The Ultrasensitive Detection of Aflatoxin M1 Using Gold Nanoparticles Modified Electrode with Fe3+ as a Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
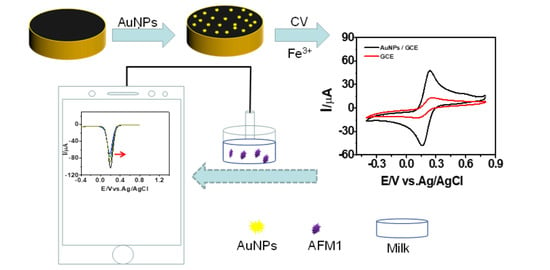
2.2. Preparation of AuNPs/GCE
2.3. Sample Preparation
3. Results and Discussion
3.1. Electrochemical Characterization of the AuNPs/GCE
3.2. Effect of pH and Scan Rates on Fe3+ Signals at AuNPs/GCE in the Presence of AFM1
3.3. Electrochemical Response of Fe3+ at AuNPs/GCE in the Presence of AFM1
3.4. Chronoamperometric Studies
3.5. Effect of Fe3+ Concentration on AFM1 Fluorescence Intensity
3.6. Determination of AFM1 in Milk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, H.H.; Sun, J.Z.; Zhang, Y.; Bian, C.; Xia, S.H.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Srivastava, S.; Ali, M.A.; Singh, C. Nanomaterial-based biosensors for food toxin detection. Appl. Biochem. Biotechnol. 2014, 174, 880–896. [Google Scholar] [CrossRef]
- Machida, M.; Gomi, K. Aspergillus: Molecular Biology and Genomics; Horizon Scientific Press: Tsukuba, Japan, 2010. [Google Scholar]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chang, C.C.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F. Perspective: Time to face the fungal threat. Nature 2014, 516, S7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Her, J.Y.; Lee, K.G. Reduction of aflatoxins (B1, B2, G1, and G2) in soybean-based model systems. Food Chem. 2015, 189, 45–51. [Google Scholar] [CrossRef]
- Turna, N.S.; Wu, F. Aflatoxin M1 in milk: A global occurrence, intake, & exposure assessment. Trends Food Sci. Tech. 2021, 110, 183–192. [Google Scholar]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Stroka, J.; Anklam, E. Development of a simplified densitometer for the determination of aflatoxins by thin-layer chromatography. J. Chromatogr. A 2000, 904, 263–268. [Google Scholar] [CrossRef]
- Kolosova, A.Y.; Shim, W.B.; Yang, Z.Y.; Eremin, S.A.; Chung, D.H. Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B1 stabilization of ELISA kit components and application to grain samples. Anal. Bioanal. Chem. 2006, 384, 286–294. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Yan, C.B.; Hashim, N.H.; Ali, A.S.M.; Salleh, M.I.; Salleh, B. Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chem. 2010, 118, 882–886. [Google Scholar] [CrossRef]
- Kav, K.; Col, R.; Tekinsen, K.K. Detection of aflatoxin M1 levels by ELISA in white-brined Urfa cheese consumed in Turkey. Food Control 2011, 22, 1883–1886. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Zhou, L.; Wang, Y.; Zhao, Y.J.; Xing, F.G.; Dai, X.F.; Liu, Y. Mycotoxin detection-recent trends at global level. Integr. Agric. 2015, 14, 2265–2281. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Emrani, A.S.; Zolfaghari, R.; Taghdisi, S.M. A new amplified π-shape electrochemical aptasensor for ultrasensitive detection of aflatoxin B1. Biosens. Bioelectron. 2017, 94, 374–379. [Google Scholar] [CrossRef]
- Ahamadi, S.F.; Hojjatoleslamy, M.; Kiani, H.; Molavi, H. Monitoring of aflatoxin M1 in milk using a novel electrochemical aptasensor based on reduced grapheme oxide and gold nanoparticles. Food Chem. 2021, 373, 131321. [Google Scholar] [CrossRef]
- Shadjou, R.; Hasanzadeh, M.; Heidar-poor, M.; Shadjou, N. Electrochemical monitoring of aflatoxin M1 in milk samples using silver nanoparticles dispersed on α-cyclodextrin-GQDs nanocomposite. J. Mol. Recognit. 2018, 31, e2699. [Google Scholar] [CrossRef]
- Perez-Fernandez, B.; Escosura-Muniz, A.D.L. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Nanomodified screen-printed electrode for direct determination of aflatoxin B1 in malted barley samples. Sens. Actuator B Chem. 2020, 307, 127547. [Google Scholar] [CrossRef]
- Suzuki, Y.; Komatsu, H.; Ikeda, T.; Satio, N.; Araki, S.; Citterio, D.; Hisamoto, D.; Kitamura, Y.; Kubota, T.; Nakagawa, J.; et al. Design and Synthesis of Mg2+ Selective Fluoroionophores Based on a Coumarin Derivative and Application for Mg2+ Measurement in a Living Cell. Anal. Chem. 2002, 74, 1423–1428. [Google Scholar] [CrossRef]
- Ciesienski, K.L.; Hyman, L.M.; Derisavifard, S.; Franz, K.J. Toward the detection of cellular copper(II) by a light-activated fluorescence increase. Inorg. Chem. 2010, 49, 6808–6810. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.R.; Feng, F.; Qiang, Y.; Liang, F.G.; Sen, L.; Wei, F.H. A coumarin-derived fluorescence chemosensors selective for copper(II). Anal. Lett. 2008, 41, 2203–2213. [Google Scholar] [CrossRef]
- Lim, N.C.; Brückner, C. DPA-substituted coumarins as chemosensors for zinc(II): Modulation of the chemosensory characteristics by variation of the position of the chelate on the coumarin. Chem. Commun. 2004, 10, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gahlyan, P.; Yadav, N.; Bhandari, M.; Kakkar, R.; Manu, D.; Prasad, A.K. Bis-triazolylated-1,4-dihydropyridin—Highly selective hydrophilic fluorescent probe for detection of Fe3+. Dyes Pigm. 2017, 147, 420–428. [Google Scholar] [CrossRef]
- Xu, Y.K.; Liu, X.G.; Zhao, J.Y.; Wang, H.Y.; Liu, Z.; Yang, X.F.; Pei, M.S.; Zhang, G.Y. A new “ON–OFF–ON” fluorescent probe for sequential detection of Fe3+ and PPi based on 2-pyridin-2-ylethanamine and benzimidazo [2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid. New J. Chem. 2019, 43, 474–480. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Liu, Q.L.; Gao, Y.; Liu, H.M.; Zhao, B. A highly selective coumarin-based chemosensor for the sequential detection of Fe3+and pyrophosphate and its application in living cell imaging. Tetrahedron Lett. 2018, 59, 1860–1865. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, T.; Fang, Y.; Wang, L.Y.; Song, B.; Deng, Q.G. Two ‘turn-off’ Schiff base fluorescence sensors based on phenanthro [9,10-d]imidazole-coumarin derivatives for Fe3+ in aqueous solution. Tetrahedron Lett. 2016, 57, 4417–4423. [Google Scholar] [CrossRef]
- Luna, D.M.N.; Avelino, K.Y.P.S.; Cordeiro, M.T.; Andrade, C.A.S.; Oliveira, M.D.L. Electrochemical immunosensor for dengue virus serotypes based on 4-mercaptobenzoic acid modified gold nanoparticles on self-assembled cysteine monolayers. Sens. Actuators B Chem. 2015, 220, 565–572. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Ramezani, M.; Danesh, N.M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor for detection of aflatoxin M1 based on target-induced immobilization of gold nanoparticles on the surface of electrode. Biosens. Bioelectron. 2018, 117, 487–492. [Google Scholar] [CrossRef]
- Micheli, L.; Grecco, R.; Badea, M.; Moscone, D.; Palleschi, G. An electrochemical immunosensor for aflatoxin M1 determination in milk using screen-printed electrodes. Biosens. Bioelectron. 2006, 21, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C 2013, 33, 2229–2234. [Google Scholar] [CrossRef]
- Smolko, V.; Shurpik, D.; Porfireva, A.; Evtugyn, G.; Stoikov, I.; Hianik, T. Electrochemical Aptasensor Based on Poly(Neutral red) and Carboxylated Pillar [5]arene for Sensitive Determination of Aflatoxin M1. Electroanalysis 2018, 30, 486–496. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.H. Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Dinçkaya, E.; Kınık, Ö.; Sezgintürk, M.K.; Altuğ, Ç.; Akkoca, A. Development of an impedimetric aflatoxin M1 biosensor based on a DNA probe and gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Patel-Sorrentino, N.; Mounier, S.; Benaim, J.Y. Excitation–emission fluorescence matrix to study pH influence on organic matter fluorescence in the Amazon basin rivers. Water Res. 2002, 36, 2571–2581. [Google Scholar] [CrossRef]
- Hussain, E.; Li, Y.X.; Cheng, C.; Zhuo, H.P.; Shahzad, S.A.; Ali, S.; Ismail, M.; Qi, H.; Yu, C. Benzo[ghi]perylene and coronene as ratiometric fluorescence probes for the selective sensing of nitroaromatic explosives. Talanta 2020, 207, 120316. [Google Scholar] [CrossRef] [PubMed]








| System | Detection Limit | Linear Range | Ref. |
|---|---|---|---|
| A-CD-GQDs-AgNPs/GCE | 2 μm | 0.015–25 μM | [17] |
| Apt-CS-AuNPs/SPGE | 0.9 ng/L | 2–600 ng/L | [29] |
| anti-AFM1/SPGE | 2.5 × 10−8 g/kg | 3 × 10−8–1.6 × 10−7 g/kg | [30] |
| Fe3O4-PANi/IDE | 1.98 ng/mL | 6–60 ng/mL | [31] |
| NR/P [5]A-COOH/GCE | 0.5 ng/L | 5–120 ng/L | [32] |
| AuNPs/SPE | 37 pg/mL | - | [33] |
| ss-HSDNA-AuNPs/GE | 0.36 ng/mL | 1–14 ng/mL | [34] |
| Fe3+-AuNPs/GCE | 1.6 × 10−21 g/L | 1.6 × 10−21–2.5 × 10−4 g/L | This work |
| Method | Add/μM | Detected/μM | Recovery% |
|---|---|---|---|
| This work | 2.96 | 2.78 | 93.9 |
| 8.91 | 8.24 | 92.4 | |
| 23.78 | 22.17 | 93.2 | |
| Spectrofluorimetry | 2.2 | 1.9 | 86 |
| 3.16 | 2.5 | 78 | |
| 12.65 | 10.08 | 84 | |
| ELISA | 2.6 | 2.39 | 92.1 |
| 8.41 | 7.82 | 93 | |
| 20.25 | 18.71 | 92.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, M.; Mo, H.; Li, H.; Xu, D.; Hu, L. The Ultrasensitive Detection of Aflatoxin M1 Using Gold Nanoparticles Modified Electrode with Fe3+ as a Probe. Foods 2023, 12, 2521. https://doi.org/10.3390/foods12132521
Li X, Zhang M, Mo H, Li H, Xu D, Hu L. The Ultrasensitive Detection of Aflatoxin M1 Using Gold Nanoparticles Modified Electrode with Fe3+ as a Probe. Foods. 2023; 12(13):2521. https://doi.org/10.3390/foods12132521
Chicago/Turabian StyleLi, Xiaobo, Miao Zhang, Haizhen Mo, Hongbo Li, Dan Xu, and Liangbin Hu. 2023. "The Ultrasensitive Detection of Aflatoxin M1 Using Gold Nanoparticles Modified Electrode with Fe3+ as a Probe" Foods 12, no. 13: 2521. https://doi.org/10.3390/foods12132521