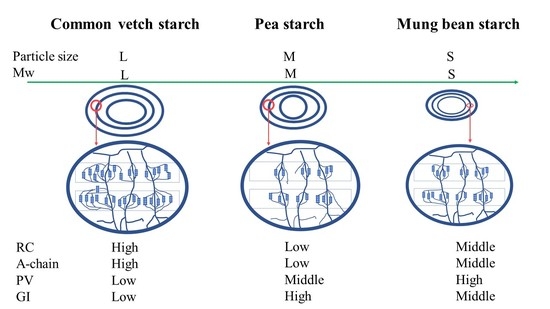
Multi-Scale Comparison of Physicochemical Properties, Refined Structures, and Gel Characteristics of a Novel Native Wild Pea Starch with Commercial Pea and Mung Bean Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Compositions
2.3. Starch Morphology and Particle Size Distribution
2.4. Molecular Weight and Chain-Length Distributions
2.5. X-ray Diffraction
2.6. Fourier Transform Infrared Spectroscopy (FT-IR) and Raman Spectroscopy
2.7. Solid State 13C CP/MAS NMR Analysis
2.8. Low-Field Nuclear Magnetic Resonance (LF-NMR) Measurement
2.9. Pasting Property and Thermal Property
2.10. Starch Solubility and Swelling Power
2.11. Gel Dynamic Rheological Properties
2.12. Gel Texture Properties
2.13. In Vitro Digestibility Analysis of Gels
2.14. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Compositions
3.2. Morphology Structure and Particle Distribution of Starch Granules
3.3. Molecular Weight and Chain-Length Distributions
3.4. Crystalline Structure
3.5. Hydrated Structure
3.6. Pasting and Thermal Properties
3.7. Rheological Properties of Starch Gels
3.8. Starch Solubility (SA), Swelling Power (SP)
3.9. Texture Properties and In Vitro Digestion of Starch Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santiago-Ramos, D.; Figueroa-Cárdenas, J.D.D.; Véles-Medina, J.J.; Salazar, R. Physicochemical properties of nixtamalized black bean (Phaseolus vulgaris L.) flours. Food Chem. 2018, 240, 456–462. [Google Scholar] [CrossRef]
- Ayalew, M. Growth and Yield Response of Mungbean (Vigna radiata L.) for the Combination of Nitrogen and Phosphorus Fertilizers with Rhizobium Strains: A Review. J. Biol. Agric. Healthc. 2019, 9, 42–48. [Google Scholar] [CrossRef]
- Liang, J.; He, Z.; Shi, W. Cotton/mung bean intercropping improves crop productivity, water use efficiency, nitrogen uptake, and economic benefits in the arid area of Northwest China. Agric. Water Manag. 2020, 240, 106227. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. A novel starch from bitter vetch (Vicia ervilia) seeds: A comparison of its physicochemical, structural, thermal, rheological, and pasting properties with conventional starches. Int. J. Food Sci. Technol. 2022, 57, 6833–6842. [Google Scholar] [CrossRef]
- Ahituv, H.; Henry, A.G. An initial key of starch grains from edible plants of the Eastern Mediterranean for use in identifying archaeological starches. J. Archaeol. Sci. Rep. 2022, 42, 103396. [Google Scholar] [CrossRef]
- Punia, S.; Dhull, S.B.; Sandhu, K.S.; Kaur, M. Faba bean (Vicia faba) starch: Structure, properties, and in vitro digestibility—A review. Legum. Sci. 2019, 1, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Worqlul, A.W.; Dile, Y.T.; Bezabih, M.; Adie, A.; Srinivasan, R.; Lefore, N.; Clarke, N. Identification of suitable areas for fodder production in Ethiopia. Catena 2022, 213, 106154. [Google Scholar] [CrossRef]
- Fu, L.; Liu, L.; Chen, W.; Wang, Q.; Lv, X.; Wang, J.; Ji, Z.; Yu, G.; Liu, Q.; Zhang, X. Physicochemical and functional characteristics of starches from common vetch (Vicia sativa L.). LWT 2020, 131, 109694. [Google Scholar] [CrossRef]
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- FAO. About FAO; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 342. [Google Scholar]
- Li, R.; Zhang, Z.; Tang, W.; Huang, Y.; Coulter, J.A.; Nan, Z. Common vetch cultivars improve yield of oat row intercropping on the Qinghai-Tibetan plateau by optimizing photosynthetic performance. Eur. J. Agron. 2020, 117, 126088. [Google Scholar] [CrossRef]
- Sayar, M.S. Path coefficient and correlation analysis between forage yield and its affecting components in common vetch (Vicia sativa L.). Legum. Res. 2014, 37, 445–452. [Google Scholar] [CrossRef]
- Bet, C.D.; Cordoba, L.D.P.; Ribeiro, L.S.; Schnitzler, E. Common Vetch (Vicia sativa) as a New Starch Source: Its Thermal, Rheological and Structural Properties After Acid Hydrolysis. Food Biophys. 2016, 11, 275–282. [Google Scholar] [CrossRef]
- Nan, W.; Lw, C.; Fza, B.; Jka, B.; Jza, B. Modifying the rheological properties, in vitro digestion, and structure of rice starch by extrusion assisted addition with bamboo shoot dietary fiber. Food Chem. 2022, 375, 131900. [Google Scholar]
- Li, M.; Wang, J.; Wang, F.; Wu, M.; Wang, R.; Strappe, P.; Blanchard, C.; Zhou, Z. Insights into the multi-scale structure of wheat starch following acylation: Physicochemical properties and digestion characteristics. Food Hydrocoll. 2022, 124, 107347. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Y.; Jia, X.; Geng, D.; Bian, X.; Tang, N. Effects of Extraction Methods on Physicochemical and Structural Properties of Common Vetch Starch. Foods 2022, 11, 2920. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Xiao, W.; Li, J.; Xie, J. Effects of xanthan, guar and Mesona chinensis Benth gums on the pasting, rheological, texture properties and microstructure of pea starch gels. Food Hydrocoll. 2022, 125, 107391. [Google Scholar] [CrossRef]
- Wang, C.; He, X.-W.; Huang, Q.; Fu, X.; Liu, S. Physicochemical properties and application of micronized cornstarch in low fat cream. J. Food Eng. 2013, 116, 881–888. [Google Scholar] [CrossRef]
- Lin, L.; Guo, D.; Zhao, L.; Zhang, X.; Wang, J.; Zhang, F.; Wei, C. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, J.; Hong, Y.; Liu, G.; Zheng, J.; Gu, Z.; Zhang, P. Impact of amylose content on starch physicochemical properties in transgenic sweet potato. Carbohydr. Polym. 2015, 122, 417–427. [Google Scholar] [CrossRef]
- Tang, J.; Zou, F.; Guo, L.; Wang, N.; Zhang, H.; Cui, B.; Liu, X. The relationship between linear chain length distributions of amylopectin and the functional properties of the debranched starch-based films. Carbohydr. Polym. 2022, 279, 119012. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolymers 2010, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. New insight into starch retrogradation: The effect of short-range molecular order in gelatinized starch. Food Hydrocoll. 2021, 120, 106921. [Google Scholar] [CrossRef]
- Yin, X.; Ma, Z.; Hu, X.; Li, X.; Boye, J.I. Molecular rearrangement of Laird lentil (Lens culinaris Medikus) starch during different processing treatments of the seeds. Food Hydrocoll. 2018, 79, 399–408. [Google Scholar] [CrossRef]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A study of ordered structure in acid-modified tapioca starch by 13C CP/MAS solid-state NMR. Carbohydr. Polym. 2004, 58, 383–389. [Google Scholar] [CrossRef]
- Sya, C.; Yu, M.A.; Cl, A.; Mam, C.; Za, D.; Lw, A.; Wei, L.A.; Lei, Z. Measurement of water fractions in freeze-dried shiitake mushroom by means of multispectral imaging (MSI) and low-field nuclear magnetic resonance (LF-NMR)—ScienceDirect. J. Food Compos. Anal. 2020, 96, 103694. [Google Scholar]
- Geng, D.-H.; Lin, Z.; Liu, L.; Qin, W.; Wang, A.; Wang, F.; Tong, L.-T. Effects of ultrasound-assisted cellulase enzymatic treatment on the textural properties and in vitro starch digestibility of brown rice noodles. LWT 2021, 146, 111543. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, N.; Wang, X.; Zhao, X.; Zhang, H. Effect of pregelatinized starch on the characteristics, microstructures, and quality attributes of glutinous rice flour and dumplings. Food Chem. 2019, 283, 248–256. [Google Scholar] [CrossRef]
- Lha, B.; Sc, A.; Yy, A.; Xx, A.; Xc, A.; Wc, A. Modification in physicochemical, structural and digestive properties of pea starch during heat-moisture process assisted by pre- and post-treatment of ultrasound. Food Chem. 2021, 360, 129929. [Google Scholar]
- Benavent-Gil, Y.; Rosell, C.M. Performance of Granular Starch with Controlled Pore Size during Hydrolysis with Digestive Enzymes. Plant Foods Hum. Nutr. 2017, 72, 353–359. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33. [Google Scholar]
- García-Alonso, A.; Goñi, I.; Saura-Calixto, F. Resistant starch and potential glycaemic index of raw and cooked legumes (lentils, chickpeas and beans). Z. Für Leb. Und -Forsch. A 1998, 206, 284–287. [Google Scholar] [CrossRef]
- Sla, C.; Qla, C.; Fza, C.; Hsa, C.; Cw, B.; Xiao, G. Effect of vacuum combined ultrasound treatment on the fine structure and physiochemical properties of rice starch. Food Hydrocoll. 2022, 124, 107198. [Google Scholar]
- Dong, S.; Fang, G.; Luo, Z.; Gao, Q. Effect of granule size on the structure and digestibility of jackfruit seed starch. Food Hydrocoll. 2021, 120, 106964. [Google Scholar] [CrossRef]
- Velásquez-Barreto, F.F.; Bello-Pérez, L.A.; Nuñez-Santiago, C.; Yee-Madeira, H.; Sánchez, C.E.V. Relationships among molecular, physicochemical and digestibility characteristics of Andean tuber starches. Int. J. Biol. Macromol. 2021, 182, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansi, W.; Mushtaq, B.S.; Mahdi, A.A.; Al-Maqtari, Q.A.; Al-Adeeb, A.; Ahmed, A.; Fan, M.; Li, Y.; Qian, H.; Jinxin, L.; et al. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zhang, X.; Zhu, C.; Wang, C.; Cheng, Y.; Tang, N. Structural, physicochemical and digestive properties of rice starch modified by preheating and pullulanase treatments. Carbohydr. Polym. 2023, 313, 120866. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J.; Zhu, Q.; Yu, J.; Jin, F. Granular structure and allomorph position in C-type Chinese yam starch granule revealed by SEM, 13C CP/MAS NMR and XRD. Food Hydrocoll. 2009, 23, 426–433. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C. Effect of Simultaneous Inhibition of Starch Branching Enzymes I and IIb on the Crystalline Structure of Rice Starches with Different Amylose Contents. J. Agric. Food Chem. 2013, 61, 9930–9937. [Google Scholar] [CrossRef]
- Rondeau-Mouro, C.; Deslis, S.; Quellec, S.; Bauduin, R. Assessment of TD-NMR and Quantitative MRI Methods to Investigate the Apple Transformation Processes Used in the Cider-Making Technology. Magn. Reson. Food Sci. 2015, 127–140. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Yang, P. Suitability of LF-NMR to analysis water state and predict dielectric properties of Chinese yam during microwave vacuum drying. LWT 2019, 105, 257–264. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Bhandari, B.; Zhou, L. LF-NMR online detection of water dynamics in apple cubes during microwave vacuum drying. Dry. Technol. 2018, 36, 2006–2015. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Oyedeji, A.B.; Ogundele, O.M.; Adebo, O.A.; Njobeh, P.B.; Kayitesi, E. Infrared heating under optimized conditions enhanced the pasting and swelling behaviour of cowpea starch. Int. J. Biol. Macromol. 2021, 184, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shang, Z.; Man, J.; Liu, Q.; Zhu, C.; Wei, C. Comparison of molecular structures and functional properties of high-amylose starches from rice transgenic line and commercial maize. Food Hydrocoll. 2015, 46, 172–179. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yang, X.; Sun, J.; Zhang, Y.; Su, D.; Zhang, H.; Wang, H. Combined molecular and supramolecular structural insights into pasting behaviors of starches isolated from native and germinated waxy brown rice. Carbohydr. Polym. 2022, 283, 119148. [Google Scholar] [CrossRef]
- Tong, C.; Ahmed, S.; Pang, Y.; Zhou, X.; Bao, J. Fine structure and gelatinization and pasting properties relationships among starches from pigmented potatoes. Food Hydrocoll. 2018, 83, 45–52. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, R.; Liu, X.; Zhang, Y.; Zhang, H.; Chi, C. Impact of long-term storage on multi-scale structures and physicochemical properties of starch isolated from rice grains. Food Hydrocoll. 2022, 124, 107255. [Google Scholar] [CrossRef]
- Yousefi, A.; Razavi, S.M. Dynamic rheological properties of wheat starch gels as affected by chemical modification and concentration. Starch-Stärke 2015, 67, 567–576. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, M.; Zhang, M.; Yadav, M.P.; Jia, X.; Yin, L. Effect of wheat bran arabinoxylan on the gelatinization and long-term retrogradation behavior of wheat starch. Carbohydr. Polym. 2022, 291, 119581. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; And, R.H.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch-Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.-F.; Lu, F.; Yang, Z.; Tao, H.; Xu, Y.; Wang, H.-L. Physical modification of waxy maize starch: Combining SDS and freezing/thawing treatments to modify starch structure and functionality. Food Struct. 2022, 32, 100263. [Google Scholar] [CrossRef]
- Wang, N.; Warkentin, T.D.; Vandenberg, B.; Bing, D.J. Physicochemical properties of starches from various pea and lentil varieties, and characteristics of their noodles prepared by high temperature extrusion. Food Res. Int. 2014, 55, 119–127. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E.; Hashemi, S.M.B.; Torri, L. Effects of sucrose, isomalt and maltodextrin on microstructural, thermal, pasting and textural properties of wheat and cassava starch gel. Int. J. Biol. Macromol. 2018, 120, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Harris, P. Food Gels; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Irani, M.; Razavi, S.M.; Abdel-Aal, E.-S.M.; Hucl, P.; Patterson, C.A. Viscoelastic and textural properties of canary seed starch gels in comparison with wheat starch gel. Int. J. Biol. Macromol. 2019, 124, 270–281. [Google Scholar] [CrossRef]
- Nie, H.; Li, C.; Liu, P.-H.; Lei, C.-Y.; Li, J.-B. Retrogradation, gel texture properties, intrinsic viscosity and degradation mechanism of potato starch paste under ultrasonic irradiation. Food Hydrocoll. 2019, 95, 590–600. [Google Scholar] [CrossRef]
- Kowittaya, C.; Lumdubwong, N. Molecular weight, chain profile of rice amylopectin and starch pasting properties. Carbohydr. Polym. 2014, 108, 216–223. [Google Scholar] [CrossRef]
- Gong, B.; Cheng, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Martinez, M.M.; Li, C.; Okoniewska, M.; Mukherjee, I.; Vellucci, D.; Hamaker, B. Slowly digestible starch in fully gelatinized material is structurally driven by molecular size and A and B1 chain lengths. Carbohydr. Polym. 2018, 197, 531–539. [Google Scholar] [CrossRef]
- Zhang, G.; Ao, Z.; Hamaker, B.R. Nutritional Property of Endosperm Starches from Maize Mutants: A Parabolic Relationship between Slowly Digestible Starch and Amylopectin Fine Structure. J. Agric. Food Chem. 2008, 56, 4686–4694. [Google Scholar] [CrossRef]







| Mung Bean Starch | Pea Starch | Common Vetch Starch | |
|---|---|---|---|
| Total starch | 91.22 ± 0.27 a | 91.79 ± 0.19 a | 92.56 ± 0.39 a |
| Moisture | 4.71 ± 0.03 b | 5.67 ± 0.03 a | 4.85 ± 0.05 b |
| Lipid | 0.93 ± 0.021 a | 0.93 ± 0.01 a | 0.77 ± 0.12 b |
| Protein | 0.93 ± 0.12 a | 1.03 ± 0.09 a | 0.83 ± 0.12 a |
| Amylose | 29.98 ± 0.22 b | 27.46 ± 0.54 c | 35.69 ± 0.54 a |
| Mung Bean Starch | Pea Starch | Common Vetch Starch | |
|---|---|---|---|
| D(4,3) | 17.49 ± 0.03 c | 26.35 ± 0.01 b | 29.28 ± 0.03 a |
| D(3,2) | 9.16 ± 0.01 c | 13.58 ± 0.01 b | 26.44 ± 0.02 a |
| D10 | 9.54 ± 0.01 c | 15.42 ± 0.01 b | 18.25 ± 0.02 a |
| D50 | 17.07 ± 0.02 c | 25.91 ± 0.01 b | 27.85 ± 0.02 a |
| D90 | 27.10 ± 0.05 c | 39.67 ± 0.03 b | 42.42 ± 0.04 a |
| Mw (kDa) | 39,310 ± 89 b | 24,996 ± 57 c | 44,042 ± 149 a |
| Mn(kDa) | 11,250 ± 244 a | 10,625 ± 192 b | 12,309 ± 128 c |
| Mw/Mn | 3.50 ± 0.08 a | 2.35 ± 0.05 b | 3.58 ± 0.03 a |
| Rz (nm) | 138 ± 1.4 b | 116 ± 0.5 c | 149 ± 0.3 a |
| DP 6–12 (%) | 35.05 ± 0.11 b | 33.76 ± 0.37 c | 37.62 ± 0.04 a |
| DP 13–24 (%) | 45.58 ± 1.38 a | 44.86 ± 1.23 a | 40.84 ± 0.05 b |
| DP 25–36 (%) | 11.62 ± 0.36 a | 12.25 ± 0.20 a | 11.96 ± 0.01 a |
| DP > 37 (%) | 7.76 ± 0.94 b | 9.11 ± 0.65 a | 9.58 ± 0.01 a |
| Mung Bean Starch | Pea Starch | Common Vetch Starch | |
|---|---|---|---|
| XRD | |||
| Crystal type | C | C | C |
| RC1 (%) | 31.49 ± 0.70 b | 27.61 ± 0.29 c | 34.16 ± 0.20 a |
| FT-IR | |||
| DO (1047 cm−1/1022 cm−1) | 1.39 ± 0.00 a | 1.33 ± 0.00 b | 1.41 ± 0.01 a |
| DD (995 cm−1/1022 cm−1) | 0.88 ± 0.00 a | 0.77 ± 0.00 b | 0.87 ± 0.01 a |
| Raman spectrum | |||
| FWHM (480 cm−1) | 29.68 ± 0.70 b | 32.29 ± 0.41 a | 24.19 ± 0.60 c |
| 13C-NMR | |||
| C1 (ppm) | 103.25, 100.65, 99.01 | 103.93, 100.80, 99.19 | 103.43, 101.15, 99.22 |
| C2, 3, 5 (ppm) | 76.19, 74.06, 71.55 | 75.52, 73.67, 71.55 | 76.13, 74.39, 71.60 |
| C4 (ppm) | 81.56 | 81.23 | 81.27 |
| RC2 (%) | 43.8 ± 1.56 b | 36.35 ± 1.23 c | 51.29 ± 1.11 a |
| DH (%) | 59.14 ± 0.86 b | 55.64 ± 0.77 c | 62.88 ± 1.03 a |
| PPA (%) | 6.54 ± 0.31 b | 10.13 ± 1.02 a | 4.72 ± 0.22 c |
| LF-NMR | |||
| A21 | 3712 ± 76 b | 6300 ± 280 a | 2689 ± 71 c |
| T21 (%) | 99.12 ± 0.6 a | 99.76 ± 0.4 a | 98.21 ± 0.04 b |
| A22 | 32.73 ± 2.12 b | 6.73 ± 0.32 c | 48.96 ± 0.01 a |
| T22 (%) | 0.87 ± 0.04 b | 0.11 ± 0.06 c | 1.79 ± 0.04 a |
| A23 | 0 | 8.19 ± 0.18 | 0 |
| T23 (%) | 0 | 0.13 ± 0.02 | 0 |
| Mung Bean Starch | Pea Starch | Common Vetch Starch | |
|---|---|---|---|
| PV (cP) | 4461 ± 45 a | 2770 ± 31 b | 1905 ± 17 c |
| TV (cP) | 2504 ± 25 a | 2100 ± 23 b | 1553 ± 14 c |
| BD (cP) | 1957 ± 20 a | 670 ± 8 b | 352 ± 3 c |
| FV (cP) | 4289 ± 43 a | 3969 ± 44 b | 2762 ± 25 c |
| SB (cP) | 1784 ± 18 b | 1869 ± 21 a | 1208 ± 11 c |
| PT (°C) | 75.92 ± 0.25 a | 74.3 ± 0.07 b | 75.6 ± 0.12 a |
| To (°C) | 55.72 ± 0.28 a | 56.75 ± 0.13 b | 57.46 ± 0.14 c |
| Tp (°C) | 68.79 ± 0.09 a | 68.66 ± 0.54 a | 67.59 ± 0.99 a |
| Tc (°C) | 83.02 ± 0.64 a | 77.04 ± 0.71 c | 80.50 ± 0.19 b |
| ΔH (J/g) | 17.43 ± 0.23 b | 15.00 ± 0.99 c | 20.17 ± 0.31 a |
| SA (%) | 13.84 ± 0.18 a | 12.66 ± 0.26 b | 14.03 ± 0.23 a |
| SP | 14.56 ± 0.01 a | 11.19 ± 0.12 b | 8.11 ± 0.07 c |
| Mung Bean Starch | Pea Starch | Common Vetch Starch | |
|---|---|---|---|
| Hardness (N) | 11960 ± 95 a | 4201 ± 46 c | 4536 ± 86 b |
| Springiness | 6.35 ± 0.05 a | 3.78 ± 0.02 c | 5.88 ± 0.10 b |
| Cohesiveness | 0.29 ± 0.00 b | 0.23 ± 0.00 c | 0.42 ± 0.00 a |
| Gumminess | 38.12 ± 0.52 a | 7.18 ± 0.06 c | 8.53 ± 0.13 b |
| RDS (%) | 64.39 ± 0.30 b | 74.21 ± 0.04 a | 56.78 ± 0.1 c |
| SDS (%) | 22.85 ± 0.14 a | 16.22 ± 0.05 b | 22.15 ± 0.58 a |
| RS (%) | 12.75 ± 0.04 b | 9.57 ± 0.01 c | 21.07 ± 0.48 a |
| HI | 49.10 ± 0.73 b | 52.61 ± 1.22 a | 43.28 ± 1.13 c |
| GI | 66.66 ± 0.47 b | 68.59 ± 0.31 a | 63.47 ± 0.24 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tang, N.; Jia, X.; Geng, D.; Cheng, Y. Multi-Scale Comparison of Physicochemical Properties, Refined Structures, and Gel Characteristics of a Novel Native Wild Pea Starch with Commercial Pea and Mung Bean Starch. Foods 2023, 12, 2513. https://doi.org/10.3390/foods12132513
Zhang X, Tang N, Jia X, Geng D, Cheng Y. Multi-Scale Comparison of Physicochemical Properties, Refined Structures, and Gel Characteristics of a Novel Native Wild Pea Starch with Commercial Pea and Mung Bean Starch. Foods. 2023; 12(13):2513. https://doi.org/10.3390/foods12132513
Chicago/Turabian StyleZhang, Xiaojun, Ning Tang, Xin Jia, Donghui Geng, and Yongqiang Cheng. 2023. "Multi-Scale Comparison of Physicochemical Properties, Refined Structures, and Gel Characteristics of a Novel Native Wild Pea Starch with Commercial Pea and Mung Bean Starch" Foods 12, no. 13: 2513. https://doi.org/10.3390/foods12132513