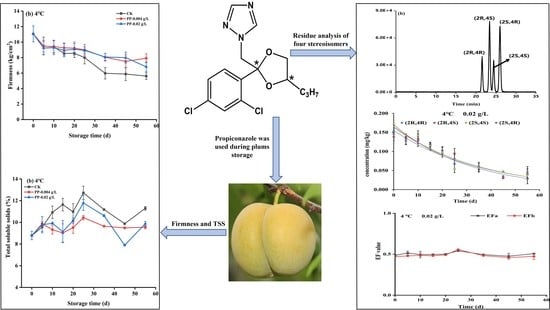
Degradation Dynamics and Residue Analysis of Four Propiconazole Stereoisomers in “Fengtang” Plum during Storage by LC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory Processing
2.3. Sample Extraction
2.4. Instrumental Methods
2.5. Fruit Firmness
2.6. Total Soluble Solids (TSS)
2.7. Data Analysis
2.7.1. Dissipation Kinetics
2.7.2. Matrix Effect
2.7.3. The Enantiomer Fraction (EF) Calculated
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extractants and Purify Agents
3.2. Method Verification Result
3.3. Dissipation Kinetics of Propiconazole Enantiomers in Plum
3.4. Final Residues of Propiconazole Enantiomers in Plum
3.5. Effect of Cleaning on Propiconazole Total Residue in Plum
3.6. Changes in the Firmness of Plums
3.7. Changes in the Total Soluble Solids Content of Plums
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Geographical Indication Agricultural Product Inquiry System. Available online: http://www.anluyun.com (accessed on 10 March 2023).
- Song, Z.F.; Ao, Y.F.; Zhang, L.; Zhong, S.L.; Chen, H.Y.; Song, S.Y.; Li, Y.Q. Study on pollen viability of melittin plum. Tillage Cultiv. 2020, 40, 48–49. [Google Scholar]
- Dong, X.Q.; Shi, Q.Y.; Lu, M. Effects of oxalic acid treatment on storage quality of harvest fengtang plum. Food Sci. Technol. 2020, 45, 53–59. [Google Scholar]
- Wang, X.H. The Sugar-Acid Components of ‘Fengtang Plum’ Fruit and the Regularity of Them Accumulation. Master’s Thesis, Guizhou University, Guiyang, China, 2018. [Google Scholar]
- Huang, S.A.; Dong, X.Q.; Cai, M.J.; Lin, X.; Zhang, Q.; Zhu, S.L. Effects of melatonin treatment on fruit quality and physiological characteristics of postharvest “fengtang plum”. N. Hortic. 2021, 489, 103–110. [Google Scholar]
- Zhang, W.; Wu, Z.Y.; Luo, L.; Luo, A.M.; Zhang, W.X. Inhibitory effect of medicinal and edible plant extracts on common rot-causing fungi in post-harvest plums. China Brew. 2019, 38, 166–169. [Google Scholar]
- Fabrício, P.G.; Marise, C.M.; Geraldo, J.S.J.; Silvia, A.L.; Lilian, A. Postharvest control of brown rot and Rhizopus rot in plums and nectarines using carnauba wax. Postharvest Biol. Technol. 2010, 58, 211–217. [Google Scholar]
- Zhang, D.P.; Davide, S.; Angelo, G.; Maria, L.G. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control. 2010, 54, 172–180. [Google Scholar] [CrossRef]
- Sun, X.T.; Cao, J.K.; Chen, N. Effects of yeast mannan treatment on storage characteristics and brown rot disease of plum fruits. Food Sci. 2011, 32, 261–264. [Google Scholar]
- Tang, S.Y.; Wang, F.; Meng, X.R.; Zhang, Y.P. Research progress in chromatographic separation of triazole fungicides containing two chiral centers. Chin. J. Pestic. Sci. 2021, 23, 617–627. [Google Scholar]
- Song, Q.M.; Wang, Y.; Tang, S.Y.; Meng, X.R.; Wang, F.; Hu, D.Y.; Zhang, Y.P. Enantioselective analysis and degradation studies of four stereoisomers of difenoconazole in citrus by chiral liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2021, 69, 501–510. [Google Scholar] [CrossRef]
- China Pesticide Information Network. Crop Registration Information. Available online: http://www.chinapesticide.org.cn/yxcftozw.jhtml (accessed on 10 March 2023).
- McKay, A.H.; Förster, H.; Adaskaveg, J.E. Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef]
- Charpe, A.M.; Borkar, P.A.; Ingole, M.N. Management of postharvest sour rot of Nagpur mandarin incited by geotrichum candidum. J. Plant Dis. Sci. 2017, 12, 23–28. [Google Scholar]
- Chen, D.; Förster, H.; Adaskaveg, J.E. Natamycin, a biofungicide for managing major postharvest fruit decays of citrus. Plant Dis. 2021, 105, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Saito, S.; Xiao, C.L. Fungicide resistance of alternaria alternata and A. arborescens isolates from Mandarin Fruit and Its Influence on control of postharvest alternaria rot. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Propiconazole [EB/OL]. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-122101_18-Jul-06.pdf (accessed on 12 May 2023).
- Ge, J. Study on the Ecotoxicological Effect Toxicity of Two Kinds of Triazole Fungicides on Zebrafish (Danio rerio). Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2018. [Google Scholar]
- GB/T 2763-2019; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. Standards Press of China: Beijing, China, 2021.
- The European Union Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 10 March 2023).
- Xu, J.; Long, X.F.; Ge, S.J.; Li, M.L.; Chen, L.Z.; Hu, D.Y.; Zhang, Y.P. Deposition amount and dissipation kinetics of difenoconazole and propiconazole applied on banana with two commercial spray adjuvants. RSC Adv. 2019, 9, 19780–19790. [Google Scholar] [CrossRef]
- Li, Z.T.; Li, H.H.; Yang, H.X.; Jian, Q.Q.; Wang, J. Determination of propiconazole pesticide pesidues in apples by QuEChERS-GC-MS/MS and matrix effect. China Food Saf. Mag. 2022, 338, 117–119+123. [Google Scholar]
- Bai, A.J.; Chen, A.; Chen, W.Y.; Luo, X.W.; Liu, S.W.; Zhang, M.; Liu, Y.; Zhang, D.Y. Study on degradation behaviour, residue distribution and dietary risk assessment of propiconazole in celery and onion under field application. J. Sci. Food Agric. 2020, 101, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jiang, W.; Jian, Q.; Song, W.C.; Zheng, Z.T.; Wang, D.L.; Liu, X.J. Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chem. 2015, 168, 396–403. [Google Scholar] [CrossRef]
- Yang, C.L.; Jiang, M.G. Separation and detection of cis and trans-isomers of propiconazole by chromatography. Chin. J. Anal. Lab. 2002, 2, 24–26. [Google Scholar]
- Tang, S.Y.; Meng, X.R.; Wang, F.; Lin, Q.; Feng, T.T.; Hu, D.Y.; Zhang, Y.P. Four propiconazole stereoisomers: Stereoselective bioactivity, separation via liquid chromatography-tandem mass spectrometry, and dissipation in banana leaves. J. Agric. Food Chem. 2022, 70, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.W.; Avants, J.K.; Miller, R.D. Loss of propiconazole and its four stereoisomers from the water phase of two soil-water slurries as measured by capillary electrophoresis. Int. J. Environ. Res. Public Health 2011, 8, 3453–3467. [Google Scholar] [CrossRef]
- Pan, X.L.; Cheng, Y.P.; Dong, F.S.; Liu, N.; Xu, J.; Liu, X.G.; Wu, X.H.; Zheng, Y.Q. Stereoselective bioactivity, acute toxicity and dissipation in typical paddy soils of the chiral fungicide propiconazole. J. Hazard. Mater. 2018, 359, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.P.; Zheng, Y.Q.; Dong, F.S.; Li, J.; Zhang, Y.F.; Sun, S.H.; Li, N.; Cui, X.Y.; Wang, Y.H.; Pan, X.L.; et al. Stereoselective analysis and dissipation of propiconazole in wheat, grapes, andsoil by supercritical fluid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.M.; Zeng, X.J.; Song, H.K.; Lin, T.; Wang, J.L.; Liu, H.C. Determination of 3 chiral fungicide pesticide residues in Pu-erh tea by multiwalled carbon nanotubes/primary secondary amine mixed adsorption-ultra performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. Insp. 2020, 11, 1702–1708. [Google Scholar]
- Zhao, H.Y.; Liu, Y.L.; Sun, Y.J.; Zhang, Z.H.; Yang, G.Q.; Wang, X.Q. Dietary risk assessment of 4 pesticides residue in bayberries and effect of houshold washing method on residue removal. J. Pestic. Sci. 2021, 23, 146–153. [Google Scholar]
- Wang, H. Study on influencing factors of pesticide residue removal test in fruits and vegetables. Food Saf. Mag. 2022, 20, 74–77. [Google Scholar]
- Wu, J.; Qiu, M.Y.; Yu, C.S.; Zhang, X.J. Research progress on pesticide residues and removal of pesticide residues in fruits and vegetables by different methods. Pestic. Sci. Adm. 2023, 44, 34–40+57. [Google Scholar]
- Wang, C.W.; Wang, Y.; Wang, R.; Yan, J.Q.; Lv, Y.B.; Li, A.J.; Gao, J. Dissipation kinetics, residues and risk assessment of propiconazole and azoxystrobin in ginseng and soil. Int. J. Environ. Anal. Chem. 2017, 97, 1–13. [Google Scholar] [CrossRef]
- Besil, N.; Cesio, V.; Heinzen, H.; Fernandez-Alba, A.R. Matrix effects and interferences of different citrus fruit coextractives in pesticide residue analysis using ultrahigh-performance liquid chromatography–high-resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 4819–4829. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H.; Deng, H.L.; Liang, R.J.; Yang, Z.F.; Su, X.G. Effects of exogenous ethylene and 1-methylcyclopropene on postharvest physicochemical and aroma characteristics of ‘Shengxin’ mango. Food Sci. 2020, 41, 231–237. [Google Scholar]
- Luo, L.; Fu, Y.S.; Chen, W.L.; Nie, Y.H.; Zhao, Y.R.; Wang, S.M. Study on the preservation effect of ultrasound-heat treatingfresh-cut Lotus root. J. Anhui Polytech. Univ. 2022, 37, 11–17. [Google Scholar]
- Li, B.X.; Yu, Y.L.; He, Y.; Guo, L.R.; Ren, D.; Xu, D. Effect of chitosan-nanocrystal cellulose composite coating on the preservation of shatangju mandarin. Food Sci. 2021, 42, 185–192. [Google Scholar]






| Matrices | Analytes | Linear Equation | SD * | Correlation R2 | Concentration (µg/mL) a | Matrix Effects (ME) | LOD b (mg/kg) | LOQ b (mg/kg) b |
|---|---|---|---|---|---|---|---|---|
| Acetonitrile | (2R,4R)-propiconazole | y = 3.92 × 106x − 55468 | 1.42 × 105 | 0.9958 | 0.02–4 | |||
| (2R,4S)-propiconazole | y = 9.21 × 106x − 37816 | 2.08 × 105 | 0.9993 | |||||
| (2S,4S)-propiconazole | y = 3.97 × 106x − 47049 | 2.02 × 104 | 0.9982 | |||||
| (2S,4R)-propiconazole | y = 9.32 × 106x − 78100 | 5.03 × 104 | 0.9986 | |||||
| Fengtang plum | (2R,4R)-propiconazole | y = 6.89 × 106x − 123123 | 2.23 × 105 | 0.9991 | 0.02–4 | 0.76 | 0.0005 | 0.004 |
| (2R,4S)-propiconazole | y = 1.54 × 107x − 311511 | 5.31 × 105 | 0.9989 | 0.67 | 0.0005 | 0.006 | ||
| (2S,4S)-propiconazole | y = 6.15 × 106x − 121224 | 9.11 × 104 | 0.9987 | 0.55 | 0.0005 | 0.004 | ||
| (2S,4R)-propiconazole | y = 1.56 × 107x − 307315 | 4.36 × 105 | 0.9991 | 0.67 | 0.0005 | 0.006 |
| Average Addition Recovery Rate (%), Intraday RSD (%, n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analytes | Add Level (mg/kg) a | Day 1 | Day 2 | Day 3 | Interday RSD (%, n = 15) | |||
| (2R,4R)-propiconazole | 0.02 | 91.16 | 4.71 | 92.13 | 1.17 | 79.42 | 3.64 | 7.23 |
| 0.20 | 96.01 | 3.17 | 76.27 | 4.65 | 99.41 | 4.69 | 11.68 | |
| 2.00 | 92.88 | 4.29 | 81.23 | 1.83 | 98.12 | 1.90 | 8.07 | |
| (2R,4S)-propiconazole | 0.02 | 94.19 | 6.71 | 95.03 | 1.98 | 82.81 | 4.51 | 7.58 |
| 0.20 | 96.58 | 4.22 | 81.11 | 3.12 | 100.27 | 5.45 | 10.12 | |
| 2.00 | 97.85 | 3.89 | 88.23 | 1.16 | 101.66 | 2.34 | 6.31 | |
| (2S,4S)-propiconazole | 0.02 | 92.28 | 5.83 | 96.86 | 1.36 | 81.19 | 4.69 | 8.21 |
| 0.20 | 95.62 | 3.98 | 86.59 | 4.31 | 104.10 | 3.40 | 8.22 | |
| 2.00 | 93.06 | 4.74 | 91.60 | 1.61 | 100.38 | 2.47 | 5.01 | |
| (2S,4R)-propiconazole | 0.02 | 91.73 | 4.04 | 95.00 | 1.51 | 80.12 | 6.84 | 8.16 |
| 0.20 | 97.29 | 2.57 | 82.41 | 1.71 | 104.05 | 4.94 | 9.90 | |
| 2.00 | 95.66 | 4.15 | 86.02 | 1.54 | 98.25 | 1.64 | 6.09 | |
| Storage Temperature 20 °C | Storage Temperature 4 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group (g/L) | Analytes | Regressive Function | R2 | T1/2 (d) | SD (d) | Regressive Function | R2 | T1/2 (d) | SD (d) |
| 0.004 | (2R,4R)-propiconazole | Ct = 0.0548e−0.055t | 0.7491 | 12.60 | 0.58 | Ct = 0.0636e−0.024t | 0.9007 | 28.88 | 2.69 |
| (2R,4S)-propiconazole | Ct = 0.0542e−0.045t | 0.7532 | 15.40 | 0.63 | Ct = 0.0751e−0.029t | 0.9365 | 23.90 | 2.35 | |
| (2S,4S)-propiconazole | Ct = 0.0683e−0.073t | 0.7588 | 9.49 | 0.61 | Ct = 0.0623e−0.025t | 0.7455 | 27.72 | 2.72 | |
| (2S,4R)-propiconazole | Ct = 0.0537e−0.052t | 0.7535 | 13.32 | 0.94 | Ct = 0.0742e−0.029t | 0.9026 | 23.90 | 1.72 | |
| 0.020 | (2R,4R)-propiconazole | Ct = 0.1330e−0.055t | 0.7489 | 12.60 | 0.96 | Ct = 0.1553e−0.028t | 0.9455 | 24.75 | 1.20 |
| (2R,4S)-propiconazole | Ct = 0.1347e−0.05t | 0.7339 | 13.86 | 1.08 | Ct = 0.1637e−0.029t | 0.8988 | 23.90 | 3.17 | |
| (2S,4S)-propiconazole | Ct = 0.165e−0.067t | 0.7923 | 10.34 | 0.46 | Ct = 0.1658e−0.029t | 0.9395 | 23.90 | 2.33 | |
| (2S,4R)-propiconazole | Ct = 0.1372e−0.053t | 0.7685 | 13.08 | 1.14 | Ct = 0.1701e−0.033t | 0.9156 | 21.00 | 0.88 | |
| Storage Temperature | Treatment Group (g/L) | Time (Days) | (2R,4R) (mg/kg) | (2R,4S) (mg/kg) | (2S,4S) (mg/kg) | (2S,4R) (mg/kg) | Total Value (mg/kg) |
|---|---|---|---|---|---|---|---|
| 20 °C | 0.004 | 10 | 0.031 ± 0.002 | 0.033 ± 0.002 | 0.035 ± 0.002 | 0.030 ± 0.002 | 0.129 |
| 20 | 0.020 ± 0.001 | 0.021 ± 0.001 | 0.020 ± 0.003 | 0.018 ± 0.001 | 0.079 | ||
| 30 | 0.006 ± 0.002 | 0.009 ± 0.003 | 0.004 ± 0.001 | 0.009 ± 0.002 | 0.026 | ||
| 0.020 | 10 | 0.078 ± 0.007 | 0.109 ± 0.007 | 0.093 ± 0.005 | 0.106 ± 0.006 | 0.386 | |
| 20 | 0.040 ± 0.002 | 0.042 ± 0.003 | 0.042 ± 0.003 | 0.038 ± 0.003 | 0.163 | ||
| 30 | 0.026 ± 0.014 | 0.035 ± 0.017 | 0.018 ± 0.010 | 0.030 ± 0.014 | 0.108 | ||
| 4 °C | 0.004 | 10 | 0.058 ± 0.003 | 0.064 ± 0.005 | 0.061 ± 0.004 | 0.065 ± 0.004 | 0.247 |
| 20 | 0.040 ± 0.008 | 0.045 ± 0.008 | 0.044 ± 0.008 | 0.044 ± 0.008 | 0.173 | ||
| 55 | 0.020 ± 0.002 | 0.018 ± 0.002 | 0.030 ± 0.003 | 0.018 ± 0.002 | 0.086 | ||
| 0.020 | 10 | 0.134 ± 0.010 | 0.131 ± 0.009 | 0.142 ± 0.014 | 0.134 ± 0.020 | 0.540 | |
| 20 | 0.094 ± 0.007 | 0.103 ± 0.010 | 0.097 ± 0.006 | 0.102 ± 0.009 | 0.397 | ||
| 55 | 0.046 ± 0.014 | 0.041 ± 0.013 | 0.038 ± 0.011 | 0.029 ± 0.014 | 0.153 |
| Treatment Group (g/L) | Samples | (2R,4R) (mg/kg) | (2R,4S) (mg/kg) | (2S,4S) (mg/kg) | (2S,4R) (mg/kg) | Total Value (mg/kg) | Reduction Ratio (%) |
|---|---|---|---|---|---|---|---|
| 0.004 | Unprocessed | 0.020 ± 0.002 | 0.018 ± 0.002 | 0.030 ± 0.003 | 0.018 ± 0.002 | 0.086 | |
| Cleaning | 0.011 ± 0.001 | 0.009 ± 0.004 | 0.011 ± 0.003 | 0.008 ± 0.001 | 0.039 | 54.65 | |
| 0.020 | Unprocessed | 0.046 ± 0.014 | 0.041 ± 0.013 | 0.038 ± 0.011 | 0.029 ± 0.014 | 0.154 | |
| Cleaning | 0.019 ± 0.010 | 0.019 ± 0.001 | 0.021 ± 0.012 | 0.019 ± 0.008 | 0.078 | 49.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Mou, L.; Ou, G.; Luo, X.; Hu, D.; Zhang, Y. Degradation Dynamics and Residue Analysis of Four Propiconazole Stereoisomers in “Fengtang” Plum during Storage by LC-MS/MS. Foods 2023, 12, 2200. https://doi.org/10.3390/foods12112200
Deng P, Mou L, Ou G, Luo X, Hu D, Zhang Y. Degradation Dynamics and Residue Analysis of Four Propiconazole Stereoisomers in “Fengtang” Plum during Storage by LC-MS/MS. Foods. 2023; 12(11):2200. https://doi.org/10.3390/foods12112200
Chicago/Turabian StyleDeng, Pengyu, Lianhong Mou, Guipeng Ou, Xin Luo, Deyu Hu, and Yuping Zhang. 2023. "Degradation Dynamics and Residue Analysis of Four Propiconazole Stereoisomers in “Fengtang” Plum during Storage by LC-MS/MS" Foods 12, no. 11: 2200. https://doi.org/10.3390/foods12112200