Third-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours: Microwave Expansion Kinetics and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
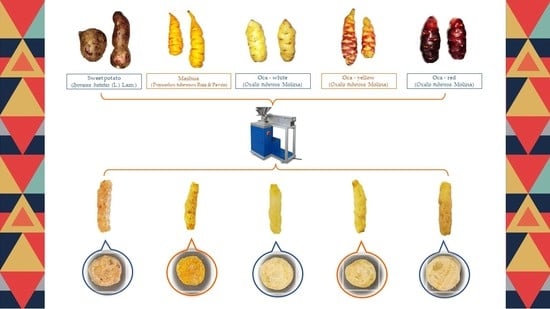
2.2. Andean Tubers and Tuberous Roots Used for Flour Manufacturing
2.3. Production of Extruded 3G Pellets
2.4. Microwave Dehydration of Dried 3G Pellets
2.4.1. Microwave Dehydration Kinetics of Dried 3G Pellets
2.4.2. Microwave Expansion Kinetics of Dried 3G Pellet
2.5. Characterization of Dried 3G Pellets and Expanded Snacks
2.5.1. Water Content of Mixture, Extruded 3G Pellets, Dried 3G Pellets, and Expanded Snacks, and Water Activity of Dried 3G Pellets
2.5.2. Hygroscopicity of Dried 3G Pellets and Expanded Snacks
2.5.3. Bulk Density and Porosity of Expanded Snacks
2.5.4. Water Absorption Index (WAI), Water Solubility Index (WSI), and Swelling Index (SWE) of Expanded Snacks
2.5.5. Texture Properties of Expanded Snacks
2.5.6. Optical Properties of Mixture, Dried 3G Pellets, and Expanded Snacks
2.5.7. Bioactive Compounds of Expanded Snack
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microwaving Dehydration Kinetics of Dried 3G Pellets; Mathematical Modeling
3.2. Sectional Expansion Index (SEI) and Appearance of Dried 3G Pellet as A Function of Microwaving Time
3.3. Characterization of Dried 3G Pellets and Expanded Snacks
3.3.1. Water Content of the Mixture, Extruded 3G Pellets, Dried 3G Pellets, and Expanded Snacks, and Water Activity of the Dried 3G Pellets
3.3.2. Bulk Density and Porosity of Expanded Snacks
3.3.3. Hygroscopicity
3.3.4. Water Absorption Index (WAI), Water Solubility Index (WSI), and Swelling Index (SWE) of Expanded Snacks
3.3.5. Texture Properties of Expanded Snacks
3.3.6. Optical Properties of Mixture, Dried 3G Pellets, and Expanded Snacks
3.3.7. Bioactive Compounds of Expanded Snacks
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns, T. A chemical–ecological model of root and tuber domestication in the Andes. In Foraging and Farming; Routledge: London, UK, 2014; pp. 504–520. [Google Scholar]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Statistics Database; FAO: Rome, Italy, 2018. [Google Scholar]
- Flores, H.E.; Walker, T.S.; Guimarães, R.L.; Bais, H.P.; Vivanco, J.M. Andean Root and Tuber Crops: Underground Rainbows. Hortscience 2003, 38, 161–167. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- Odake, K.; Hatanaka, A.; Kajiwara, T.; Muroi, T.; Nishiyama, T.; Yamakawa, O. Evaluation method and breeding of purple sweet potato “Yamagawa murasaki” (Ipomoea batatas Poir.) for raw material of food colorants. Nippon. Shokuhin Kogyo Gakkaishi 1994, 41, 287–293. [Google Scholar] [CrossRef]
- Oke, M.O.; Workneh, T.S. A review on sweet potato postharvest processing and preservation technology. Int. J. Agric. Sci. 2019, 9, 001–014. [Google Scholar]
- Grau, A.; Duenas, R.O.; Cabrera, C.N.; Herman, M. Mashua (Tropaeolum tuberosum Ruíz & Pav.). Promoting the Conservation and Use of Under-Utilized and Neglected Crops; Engels, J., Ed.; International Potato Center: Lima, Peru; International Plant Genetic Resources: Rome, Italy, 2003; Volume 25. [Google Scholar]
- Apaza Ticona, L.N.; Tena Pérez, V.; Bermejo Benito, P. Local/traditional uses, secondary metabolites and biological activities of Mashua (Tropaeolum tuberosum Ruíz & Pavón). J. Ethnopharmacol. 2020, 247, 112152. [Google Scholar]
- Leidi, E.O.; Altamirano, A.M.; Mercado, G.; Rodriguez, J.P.; Ramos, A.; Alandia, G.; Sørensen, M.; Jacobsen, S.-E. Andean roots and tubers crops as sources of functional foods. J. Funct. Foods 2018, 51, 86–93. [Google Scholar] [CrossRef]
- Collazos, Q. La Composición de los Alimentos Peruanos; Ministerio de Salud: Lima, Peru, 1975. [Google Scholar]
- Lim, T. Oxalis tuberosa. In Edible Medicinal and Non-Medicinal Plants; Springer: Cham, Switzerland, 2016; pp. 139–146. [Google Scholar]
- Zhu, F.; Cui, R. Comparison of physicochemical properties of oca (Oxalis tuberosa), potato, and maize starches. Int. J. Biol. Macromol. 2020, 148, 601–607. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Babić, J.; Jozinović, A.; Ačkar, Đ.; Miličević, B.; Muhamedbegović, B.; Šubarić, D. Production of third-generation snacks. Croat. J. Food Sci. Technol. 2018, 10, 98–105. [Google Scholar] [CrossRef]
- Tovar-Jiménez, X.; Caro-Corrales, J.; Gómez-Aldapa, C.A.; Zazueta-Morales, J.D.J.; Limón-Valenzuela, V.; Castro-Rosas, J.; Hernández-Ávila, J.; Aguilar-Palazuelos, E. Third generation snacks manufactured from orange by-products: Physicochemical and nutritional characterization. J. Food Sci. Technol. 2015, 52, 6607–6614. [Google Scholar] [CrossRef]
- Camacho-Hernández, I.; Zazueta-Morales, J.; Gallegos-Infante, J.; Aguilar-Palazuelos, E.; Guzmán, N.E.R.; Cortez, R.O.N.; Jacobo-Valenzuela, N.; Gomez-Aldapa, C. Effect of extrusion conditions on physicochemical characteristics and anthocyanin content of blue corn third-generation snacks. CyTA-J. Food 2014, 12, 320–330. [Google Scholar] [CrossRef]
- Gümüşay, A.; Şeker, M. Effects of extrusion parameters on physicochemical properties of third generation corn snacks expanded by microwave heating. J. Food Process. Preserv. 2021, 45, e15630. [Google Scholar] [CrossRef]
- Bastos-Cardoso, I.; Zazueta-Morales, J.; Martínez-Bustos, F.; Kil-Chang, Y. Development and characterization of extruded pellets of whole potato (Solanum tuberosum L.) flour expanded by microwave heating. Cereal Chem. 2007, 84, 137–144. [Google Scholar] [CrossRef]
- De Souza, V.F.; Ortiz, J.A.R.; do Nascimento, E.M.d.G.C. Pasting properties of expanded extrudate and pellets from corn flour and rice flour. Braz. J. Food Technol. 2011, 14, 106–114. [Google Scholar] [CrossRef]
- de Carvalho, R.; Ascheri, J.; Cal-Vidal, J. Effect of extrusion parameters in the physical properties of pellets (3G) from blends of wheat, rice and banana flours. Ciência E Agrotecnol. 2002, 25, 1006–1018. [Google Scholar]
- Gomez-Aldapa, C.; Navarro-Cortez, R.; Aguilar-Palazuelos, E.; Zazueta-Morales, J.; Rosas, J.C.; Hernandez-Ávila, J.; Aguirre-Tostado, F. Microstructure of an Extruded Third-Generation Snack Made from a Whole Blue Corn and Corn Starch Mixture. Int. J. Food Process. Technol. 2014, 1, 10–17. [Google Scholar] [CrossRef]
- Portela, J.B.; Coimbra, P.T.; Cappato, L.P.; Alvarenga, V.O.; Oliveira, R.B.; Pereira, K.S.; Azeredo, D.R.P.; Sant’Ana, A.S.; Nascimento, J.S.; Cruz, A.G. Predictive model for inactivation of salmonella in infant formula during microwave heating processing. Food Control 2019, 104, 308–312. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Acurio, L.; Moreau, F.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Microwave Expansion Kinetics of Third-Generation Extruded Corn Pellets under Different Moisture Contents. Biol. Life Sci. Forum 2022, 18, 51. [Google Scholar] [CrossRef]
- Salazar, D.; Arancibia, M.; Ocaña, I.; Rodríguez-Maecker, R.; Bedón, M.; López-Caballero, M.E.; Montero, M.P. Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador. Agronomy 2021, 11, 1693. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.-H.; Mujumdar, A.S.; Wang, D.; Zhao, J.-H.; Fang, X.-M.; Zhang, Q.; Xie, L.; Gao, Z.-J.; Xiao, H.-W. Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure and drying kinetics of red bell pepper (Capsicum annuum L.). LWT 2017, 77, 337–347. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Gachovska, T.K.; Ngadi, M.O.; Raghavan, G.S.V. Effect of Pretreatments on Drying Characteristics of Okra. Dry Technol. 2008, 26, 1251–1256. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel Ingredients Based on Grapefruit Freeze-Dried Formulations: Nutritional and Bioactive Value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.T.; Berrios, J.D.J.; Tang, J.; Swanson, B.G. Evaluation of Methods for Expansion Properties of Legume Extrudates. Appl. Eng. Agric. 2007, 23, 777–783. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Gujska, E.; Khan, K. Functional Properties of Extrudates from High Starch Fractions of Navy and Pinto Beans and Corn Meal Blended with Legume High Protein Fractions. J. Food Sci. 1991, 56, 431–435. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of insects and pea powder as alternative protein and mineral sources in extruded snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Singh, N.; Smith, A.C. A comparison of wheat starch, whole wheat meal and oat flour in the extrusion cooking process. J. Food Eng. 1997, 34, 15–32. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Influence of microalgae addition in formulation on colour, texture, and extrusion parameters of corn snacks. Food Sci. Technol. Int. 2020, 26, 685–695. [Google Scholar] [CrossRef]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Bouvier, J.M. Melt expansion in extrusion-cooking. Feed. Technol. 1997, 1, 13–15. [Google Scholar]
- Igual, M.; Chiş, M.S.; Păucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of Rose Hip (Rosa canina) Puree Co-Product in Enriched Corn Extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.O.; Hurtado, M.C.; Mata, M.S.; Ruiz, V.F.; de Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Khamis, H.; Lusweti, K.; Mwevura, H.; Nyanzi, S.; Kiremire, B.T. Quantification of Lycopene from Tomatoes and Watermelons by Using Beer-Lambert Principle. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 38, 119–133. [Google Scholar]
- Igual, M.; García-Martínez, E.; Camacho, M.D.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef] [PubMed]
- El-Aouar, Â.A.; Azoubel, P.M.; Murr, F.E.X. Drying kinetics of fresh and osmotically pre-treated papaya (Carica papaya L.). J. Food Eng. 2003, 59, 85–91. [Google Scholar] [CrossRef]
- Zarein, M.; Samadi, S.H.; Ghobadian, B. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. J. Saudi Soc. Agric. Sci. 2015, 14, 41–47. [Google Scholar] [CrossRef]
- Torki-Harchegani, M.; Ghanbarian, D.; Pirbalouti, A.G.; Sadeghi, M. Dehydration behaviour, mathematical modelling, energy efficiency and essential oil yield of peppermint leaves undergoing microwave and hot air treatments. Renew. Sustain. Energy Rev. 2016, 58, 407–418. [Google Scholar] [CrossRef]
- Evin, D. Microwave drying and moisture diffusivity of white mulberry: Experimental and mathematical modeling. J. Mech. Sci. Technol. 2011, 25, 2711–2718. [Google Scholar] [CrossRef]
- Giri, S.; Prasad, S. Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. J. Food Eng. 2007, 78, 512–521. [Google Scholar] [CrossRef]
- Gunhan, T.; Demir, V.; Hancioglu, E.; Hepbasli, A. Mathematical modelling of drying of bay leaves. Energy Convers. Manag. 2005, 46, 1667–1679. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Chiralt, A.; Fito, P. Food dehydration and product structure. Trends Food Sci. Technol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Marshall, W.E.; Chrastil, J. Interaction of food proteins with starch. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Elsevier Applied Science: Boston, MA, USA, 1992; pp. 75–97. [Google Scholar]
- Fernández-Gutiérrez, J.A.; Martín-Martínez, E.S.; Martínez-Bustos, F.; Cruz-Orea, A. Physicochemical Properties of Casein-Starch Interaction Obtained by Extrusion Process. Starch-Stärke 2004, 56, 190–198. [Google Scholar] [CrossRef]
- Ravindran, V.; Ravindran, G.; Sivakanesan, R.; Rajaguru, S.B. Biochemical and nutritional assessment of tubers from 16 cultivars of sweet potato (Lpomoea batatas L.). J. Agric. Food Chem. 1995, 43, 2646–2651. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Rondan-Sanabria, G.G.; Finardi-Filho, F. The physical, chemical and functional characterization of starches from Andean tubers: Oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón). Braz. J. Pharm. Sci. 2013, 49, 453–464. [Google Scholar] [CrossRef]
- Akdogan, H. High moisture food extrusion. Int. J. Food Sci. Technol. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H.; Reid, D.S. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef]
- Igual, M.; Moreau, F.; García-Segovia, P.; Martínez-Monzó, J. Valorization of Beetroot By-Products for Producing Value-Added Third Generation Snacks. Foods 2023, 12, 176. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Samborska, K.; Mitrus, M.; Oniszczuk, T.; Combrzyński, M.; Soja, J.; Lewko, P.; Drozd, K.K.; Oniszczuk, A. Structure and Texture Characteristics of Novel Snacks Expanded by Various Methods. Materials 2023, 16, 1541. [Google Scholar] [CrossRef]
- Jimenez, M.E.; Rossi, A.; Sammán, N. Health properties of oca (Oxalis tuberosa) and yacon (Smallanthus sonchifolius). Food Funct. 2015, 6, 3266–3274. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Konopacka, D.; Płocharski, W.; Beveridge, T. Water Sorption and Crispness of Fat-Free Apple Chips. J. Food Sci. 2002, 67, 87–92. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Kamińska-Dwórznicka, A.; Ostrowska-Ligęza, E.; Górska, A.; Wirkowska-Wojdyła, M.; Mańko-Jurkowska, D.; Górska, A.; Bryś, J. Application of Different Compositions of Apple Puree Gels and Drying Methods to Fabricate Snacks of Modified Structure, Storage Stability and Hygroscopicity. Appl. Sci. 2021, 11, 10286. [Google Scholar] [CrossRef]
- DeMan, J.M.; Finley, J.W.; Hurst, W.J.; Lee, C.Y. Principles of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 1999; Volume 1. [Google Scholar]
- Igual, M.; Baldo, J.V.L.; García-Segovia, P.; Martínez-Monzó, J. Impact of Urtica dioica on phenols, antioxidant capacity, color, texture and extrusion parameters of extruded corn products. Br. Food J. 2023, 125, 696–712. [Google Scholar] [CrossRef]
- Gomez, M.H.; Aguilera, J.M. A Physicochemical Model for Extrusion of Corn Starch. J. Food Sci. 1984, 49, 40–43. [Google Scholar] [CrossRef]
- Culețu, A.; Susman, I.E.; Mulțescu, M.; Cucu, Ș.E.; Belc, N. Corn Extrudates Enriched with Health-Promoting Ingredients: Physicochemical, Nutritional, and Functional Characteristics. Processes 2023, 11, 1108. [Google Scholar] [CrossRef]
- Jin, Z.; Hsieh, F.; Huff, H. Effects of soy fiber, salt, sugar and screw speed on physical properties and microstructure of corn meal extrudate. J. Cereal Sci. 1995, 22, 185–194. [Google Scholar] [CrossRef]
- Doğan, H.; Karwe, M.V. Physicochemical Properties of Quinoa Extrudates. Food Sci. Technol. Int. 2003, 9, 101–114. [Google Scholar] [CrossRef]
- Seth, D.; Badwaik, L.S.; Ganapathy, V. Effect of feed composition, moisture content and extrusion temperature on extrudate characteristics of yam-corn-rice based snack food. J. Food Sci. Technol. 2015, 52, 1830–1838. [Google Scholar] [CrossRef]
- Igual, M.; Chiş, M.S.; Socaci, S.A.; Vodnar, D.C.; Ranga, F.; Martínez-Monzó, J.; García-Segovia, P. Effect of Medicago sativa Addition on Physicochemical, Nutritional and Functional Characteristics of Corn Extrudates. Foods 2021, 10, 928. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Application of extrusion technology in plant food processing byproducts: An overview. Compr. Rev. Food Sci. Food Saf. 2020, 19, 218–246. [Google Scholar] [CrossRef] [PubMed]
- Ilo, S.; Berghofer, E. Kinetics of colour changes during extrusion cooking of maize grits. J. Food Eng. 1999, 39, 73–80. [Google Scholar] [CrossRef]
- Choton, S.; Gupta, N.; Bandral, J.D.; Anjum, N.; Choudary, A. Extrusion technology and its application in food processing: A review. Pharma Innov. 2020, 9, 162–168. [Google Scholar] [CrossRef]
- Campos, D.; Noratto, G.; Chirinos, R.; Arbizu, C.; Roca, W.; Cisneros-Zevallos, L. Antioxidant capacity and secondary metabolites in four species of Andean tuber crops: Native potato (Solanum sp.), mashua (Tropaeolum tuberosum Ruiz & Pavón), Oca (Oxalis tuberosa Molina) and ulluco (Ullucus tuberosus Caldas). J. Sci. Food Agric. 2006, 86, 1481–1488. [Google Scholar]
- Hussain, A.; Pu, H.; Sun, D.-W. Measurements of lycopene contents in fruit: A review of recent developments in conventional and novel techniques. Crit. Rev. Food Sci. Nutr. 2019, 59, 758–769. [Google Scholar] [CrossRef]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for Determining the Total Content of Phenolic Compounds in Plant Extracts with Folin–Denis Reagent and Folin–Ciocalteu Reagent: Modification and Comparison. Russ. J. Bioorg. Chem. 2022, 48, 1519–1525. [Google Scholar] [CrossRef]
- Catunta, D. Efecto De La Deshidratación Osmótica Sobre Los Compuestos Antioxidantes En Dos Accesiones De Mashua (Tropaeolum tuberosum R&P); Universidad Nacional Del Altiplano: Puno, Peru, 2016; p. 91. [Google Scholar]
- Pacheco, M.T.; Hernández-Hernández, O.; Moreno, F.J.; Villamiel, M. Andean tubers grown in Ecuador: New sources of functional ingredients. Food Biosci. 2020, 35, 100601. [Google Scholar] [CrossRef]
- Leermakers, E.T.; Darweesh, S.K.; Baena, C.P.; Moreira, E.M.; van Lent, D.M.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef]
- Pérez-Balladares, D.; Castañeda-Terán, M.; Granda-Albuja, M.G.; Tejera, E.; Iturralde, G.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical Composition and Antioxidant Activity of the Main Fruits, Tubers and Legumes Traditionally Consumed in the Andean Regions of Ecuador as a Source of Health-Promoting Compounds. Plant Foods Hum. Nutr. 2019, 74, 350–357. [Google Scholar] [CrossRef]







| Sample | Model | |||
|---|---|---|---|---|
| Page | Logarithmic | Midilli–Kucuk | ||
| C | Model constants | k: 0.00833 n: 1.2098 | a: 1.1289 k: 0.0186 c: −0.0561 | a: 1.0476 k: 0.0086 n: 1.2679 b: 0.0012 |
| Adj. R2 | 92.19 | 91.4 | 91.09 | |
| RMSE | 0.085 | 0.089 | 0.091 | |
| IbP | Model constants | k: 0.00562 n: 1.29293 | a: 1.2525 k: 0.0142 c: −0.1926 | a: 1.0131 k: 0.0029 n: 1.6223 b: 0.0037 |
| Adj. R2 | 93.83 | 92.31 | 94.37 | |
| RMSE | 0.065 | 0.072 | 0.062 | |
| Tt | Model constants | k: 0.00408 n: 1.68135 | a: 1.5536 k: 0.0212 c: −0.4891 | a: 1.0221 k: 0.0028 n: 1.8694 b: 0.0019 |
| Adj. R2 | 96.19 | 91.93 | 95.61 | |
| RMSE | 0.066 | 0.096 | 0.071 | |
| OtW | Model constants | k: 0.00297 n: 1.83746 | a: 1.3471 k: 0.03 c: −0.2597 | a: 1.0371 k: 0.0013 n: 2.2165 b: 0.0028 |
| Adj. R2 | 93.76 | 86.93 | 95.38 | |
| RMSE | 0.089 | 0.128 | 0.076 | |
| OtY | Model constants | k: 0.00454 n: 1.38244 | a: 1.5418 k: 0.0114 c: −0.4939 | a: 1.0141 k: 0.0032 n: 0.9881 b: −0.0101 |
| Adj. R2 | 95.67 | 94.51 | 94.83 | |
| RMSE | 0.053 | 0.06 | 0.058 | |
| OtR | Model constants | k: 0.02928 n: 0.903809 | a: 0.8212 k: 0.0381 c: 0.2443 | a: 1.0363 k: 0.0088 n: 1.4406 b: 0.0049 |
| Adj. R2 | 87.21 | 88.56 | 90.62 | |
| RMSE | 0.09 | 0.085 | 0.077 | |
| Sample | awP | HyP | HyE | ρb | ε | WAI | WSI | SWE |
|---|---|---|---|---|---|---|---|---|
| (gw/100 gdry solid) | (gw/100 gdry solid) | (g/cm3) | % | % | (mLswollen/gdry solid) | |||
| C | 0.303 (0.003) d | 4.05 (0.03) Bc | 9.80 (0.49) Ac | 0.15 (0.0005) a | 77.34 (1.92) a | 6.18 (0.12) a | 2.06 (0.25) b | 5.00 (0.26) d |
| IbP | 0.233 (0.003) e | 6.27 (0.35) Bab | 15.81 (1.23) Aab | 0.20 (0.03) a | 73.35 (3.91) a | 5.34 (0.26) b | 4.04 (0.74) ab | 6.69 (0.29) abc |
| Tt | 0.348 (0.003) b | 6.57 (0.14) Bab | 13.22 (0.72) Aab | 0.21 (0.03) a | 84.16 (2.13) a | 4.69 (0.31) b | 3.86 (0.78) ab | 5.37 (0.06) cd |
| OtW | 0.314 (0.003) c | 5.20 (0.05) Bab | 14.35 (1.34) Aab | 0.23 (0.01) a | 71.04 (0.65) a | 5.19 (0.11) b | 4.05 (0.23) ab | 8.04 (0.59) a |
| OtY | 0.37 (0.003) a | 5.96 (0.12) Ba | 16.16 (1.99) Aa | 0.23 (0.04) a | 72.19 (5.60) a | 5.02 (0.11) b | 4.08 (0.27) ab | 6.29 (0.10) bcd |
| OtR | 0.315 (0.003) c | 6.10 (0.08) Bb | 12.92 (0.20) Ab | 0.18 (0.02) a | 74.79 (5.16) a | 5.22 (0.18) b | 4.33 (0.48) a | 7.46 (0.53) ab |
| Sample | Wc | Fs | Fp | Nsr | N0 |
|---|---|---|---|---|---|
| (N × mm) | (N) | (N) | (mm−1) | ||
| C | 0.82 (0.07) a | 6.0 (0.8) a | 5.1 (0.3) a | 7.3 (0.7) a | 57 (6) abc |
| IbP | 0.57 (0.12) b | 4.3 (0.8) b | 3.6 (0.9) b | 7.8 (0.8) a | 61 (4) a |
| Tt | 0.41 (0.09) b | 3.1 (0.6) c | 2.4 (0.4) c | 7.5 (0.7) a | 51 (7) bc |
| OtW | 0.46 (0.08) b | 3.9 (0.9) bc | 3.4 (0.9) b | 7.7 (0.6) a | 59 (4) ab |
| OtY | 0.50 (0.03) b | 3.9 (0.7) bc | 3.3 (0.6) bc | 7.5 (0,8) a | 50 (6) c |
| OtR | 0.78 (0.13) a | 5.6 (0.7) a | 4.9 (0.7) a | 7.4 (0.9) a | 54 (8) abc |
| Sample | L* | a* | b* | C* | h* | ΔE1 | ΔE2 |
|---|---|---|---|---|---|---|---|
| Mixtures before extrusion | |||||||
| Control | 77.93 (0.32) Aa | 6.72 (0.70) Cd | 42.38 (2.09) Bc | 42.91 (2.17) Bc | 81.01 (0.51) Bc | ||
| IbP | 73.45 (1.02) Ae | 4.61 (0.26) Cb | 19.05 (1.58) Be | 19.59 (1.60) Be | 76.39 (0.36) Bf | ||
| Tt | 69.27 (1.31) Af | 9.93 (1.27) Ca | 43.03 (4.75) Ba | 44.16 (4.92) Ba | 77.03 (0.22) Be | ||
| OtW | 74.39 (2.72) Ac | 6.01 (0.58) Cc | 34.85 (5.43) Bb | 35.36 (5.45) Bb | 80.16 (0.56) Bb | ||
| OtY | 76.39 (0.21) Ad | 6.45 (0.05) Cb | 41.11 (0.04) Bab | 41.61 (0.05) Bb | 81.09 (0.06) Bd | ||
| OtR | 80.63 (1.15) Ab | 4.29 (0.30) Ce | 26.17 (2.23) Bd | 26.52 (2.25) Bd | 80.68 (0.14) Ba | ||
| Dried 3G pellets | |||||||
| Control | 65.39 (1.18) Ca | 7.51 (0.49) Ad | 25.56 (2.14) Bc | 26.64 (2.19) Bc | 73.60 (0.33) Cc | ||
| IbP | 41.16 (0.26) Ce | 10.57 (0.08) Ab | 25.87 (0.23) Be | 27.94 (0.25) Be | 67.77 (0.03) Cf | ||
| Tt | 38.62 (0.00) Cf | 13.59 (0.01) Aa | 32.67 (0.00) Ba | 35.38 (0.00) Ba | 67.42 (0.01) Ce | ||
| OtW | 49.39 (0.10) Cc | 9.74 (0.005) Ac | 41.59 (0.22) Bb | 42.71 (0.21) Bb | 76.82 (0.07) Cb | ||
| OtY | 48.57 (0.17) Cd | 11.14 (0.01) Ab | 38.46 (0.15) Bab | 40.04 (0.14) Bb | 73.85 (0.07) Cd | ||
| OtR | 49.53 (0.00) Cb | 7.90 (0.01) Ae | 38.28 (0.005) Bd | 39.09 (0.004) Bd | 78.34 (0.009) Ca | ||
| Expanded snacks | |||||||
| Control | 79.56 (0.09) Ba | 3.89 (0.04) Bd | 37.35 (0.25) Ac | 37.55 (0.25) Ac | 84.06 (0.02) Ac | 73.06 (0.58) a | 74.40 (0.68) a |
| IbP | 63.77 (0.02) Be | 7.87 (0.005) Bb | 24.84 (0.01) Ae | 26.06 (0.01) Ae | 72.43 (0.02) Af | 60.25 (0.58) d | 57.82 (0.17) d |
| Tt | 63.14 (0.005) Bf | 14.00 (0.005) Ba | 49.36 (0.005) Aa | 51.31 (0.01) Aa | 74.17 (0.005) Ae | 54.10 (1.95) e | 57.76 (0.005) d |
| OtW | 76.52 (0.01) Bc | 4.43 (0.01) Bc | 38.19 (0.01) Ab | 38.44 (0.01) Ab | 83.39 (0.01) Ab | 70.82 (0.66) b | 72.17 (0.03) b |
| OtY | 71.48 (0.02) Bd | 5.89 (0.005) Bb | 39.20 (0.01) Aab | 39.63 (0.01) Ab | 81.46 (0.01) Ad | 65.25 (0.06) c | 64.55 (0.06) c |
| OtR | 74.97 (0.03) Bb | 3.77 (0.02) Be | 31.99 (0.05) Ad | 32.21 (0.06) Ad | 83.27 (0.02) Aa | 71.17 (0.59) ab | 72.00 (0.005) b |
| Sample | TC | LP | TP | AC |
|---|---|---|---|---|
| (mgβ-carotene/100 g) | (mg/100 g) | (mgGA/100 g) | (mgTrolox/100 g) | |
| C | 2.00 (0.02) d | 0.974 (0.007) b | 110.9 (1.3) b | 2.4 (0.2) d |
| IbP | 2.11 (0.03) cd | 1.07 (0.03) b | 128 (2) a | 8.18 (0.09) b |
| Tt | 7.21 (0.08) a | 1.68 (0.06) a | 129 (3) a | 9.3 (1.2) a |
| OtW | 2.18 (0.06) c | 1.02 (0.05) b | 115.9 (1.2) b | 5.5 (0.3) c |
| OtY | 3.11 (0.09) b | 1.04 (0.07) b | 116 (3) b | 5.38 (0.05) c |
| OtR | 2.12 (0.05) cd | 0.995 (0.004) b | 117 (2) b | 5.78 (0.09) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acurio, L.; Salazar, D.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Third-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours: Microwave Expansion Kinetics and Characterization. Foods 2023, 12, 2168. https://doi.org/10.3390/foods12112168
Acurio L, Salazar D, García-Segovia P, Martínez-Monzó J, Igual M. Third-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours: Microwave Expansion Kinetics and Characterization. Foods. 2023; 12(11):2168. https://doi.org/10.3390/foods12112168
Chicago/Turabian StyleAcurio, Liliana, Diego Salazar, Purificación García-Segovia, Javier Martínez-Monzó, and Marta Igual. 2023. "Third-Generation Snacks Manufactured from Andean Tubers and Tuberous Root Flours: Microwave Expansion Kinetics and Characterization" Foods 12, no. 11: 2168. https://doi.org/10.3390/foods12112168