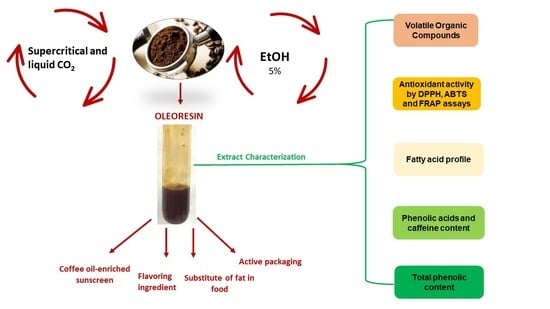
The Use of Carbon Dioxide as a Green Approach to Recover Bioactive Compounds from Spent Coffee Grounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Moisture Content
2.4. Extraction Methods
2.5. Total Polyphenol Content
2.6. Individual Polyphenols and Caffeine Assessed by Ultrahigh-Performance Liquid Chromatography Analysis
2.7. Fatty Acid Profile
2.8. Antioxidant Activity Assay
2.9. Volatile Organic Compounds
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yields
3.2. Polyphenol and Caffeine Content
3.3. Fatty Acid Composition
3.4. Antioxidant Activity
3.5. Volatile Organic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, M.; Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Chmiel, M.J. Effect of the Addition of Biochar and Coffee Grounds on the Biological Properties and Ecotoxicity of Composts. Waste Biomass Valorization 2018, 9, 1389–1398. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing Nutritional Qualities, Biological Activities and Technological Properties of Coffee Byproducts in Functional Food Formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-Value Molecules Recovery and Biofuels Production from Spent Coffee Grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent Coffee Grounds as a Valuable Source of Phenolic Compounds and Bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From Coffee Industry Waste Materials to Skin-Friendly Products with Improved Skin Fat Levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Fiore, A.G.; Caporizzi, R. How Much Caffeine in Coffee Cup? Effects of Processing Operations, Extraction Methods and Variables. In The Question of Caffeine; Latosinska, J.N., Latosinska, M., Eds.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3273-8. [Google Scholar]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In Vitro Health Promoting Properties of Antioxidant Dietary Fiber Extracted from Spent Coffee (Coffee arabica L.) Grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef]
- Shahabi Mohammadabadi, S.; Goli, M.; Naji Tabasi, S. Optimization of Bioactive Compound Extraction from Eggplant Peel by Response Surface Methodology: Ultrasound-Assisted Solvent Qualitative and Quantitative Effect. Foods 2022, 11, 3263. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent Developments on Polyphenol—Protein Interactions: Effects on Tea and Coffee Taste, Antioxidant Properties and the Digestive System. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Do Coffee Polyphenols Have a Preventive Action on Metabolic Syndrome Associated Endothelial Dysfunctions? An Assessment of the Current Evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.C.; Vu, Q.T.; Huynh, L.; Lin, C.-H.; Alvarez, S.; Vo, X.T.; Nguyen, T.H.D. Evaluation of Fatty Acids, Phenolics and Bioactivities of Spent Coffee Grounds Prepared from Vietnamese Coffee. Int. J. Food Prop. 2021, 24, 1548–1558. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and Coffee Polyphenols and Their Biological Properties Based on the Latest in Vitro Investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef] [PubMed]
- Meerasri, J.; Sothornvit, R. Novel Development of Coffee Oil Extracted from Spent Coffee Grounds as a Butter Substitute in Bakery Products. J. Food Process. Preserv. 2022, 46, e16687. [Google Scholar] [CrossRef]
- Heng, M.Y.; Tan, S.N.; Yong, J.W.H.; Ong, E.S. Emerging Green Technologies for the Chemical Standardization of Botanicals and Herbal Preparations. TrAC Trends Anal. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Leila, M.; Ratiba, D.; Al-Marzouqi, A.-H. Experimental and Mathematical Modelling Data of Green Process of Essential Oil Extraction: Supercritical CO2 Extraction. Mater. Today Proc. 2022, 49, 1023–1029. [Google Scholar] [CrossRef]
- Muangrat, R.; Pongsirikul, I. Recovery of Spent Coffee Grounds Oil Using Supercritical CO2: Extraction Optimisation and Physicochemical Properties of Oil. CyTA J. Food 2019, 17, 334–346. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Rossi, D.; Pizzolongo, F.; Masi, P. Bioactive Compounds Extracted by Liquid and Supercritical Carbon Dioxide from Citrus Peels. Int. J. Food Sci. Technol. 2022, 57, 3826–3837. [Google Scholar] [CrossRef]
- Romano, R.; Aiello, A.; Meca, G.; De Luca, L.; Pizzolongo, F.; Masi, P. Recovery of Bioactive Compounds from Walnut (Juglans regia L.) Green Husk by Supercritical Carbon Dioxide Extraction. Int. J. Food Sci. Technol. 2021, 56, 4658–4668. [Google Scholar] [CrossRef]
- Moret, S.; Purcaro, G.; Conte, L. Il Campione Per L’analisi Chimica: Tecniche Innovative e Applicazioni Nei Settori Agroalimentare e Ambientale; Springer: Milan, Italy, 2014; ISBN 978-88-470-5738-8. [Google Scholar]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical Fluid Extraction from Spent Coffee Grounds and Coffee Husks: Antioxidant Activity and Effect of Operational Variables on Extract Composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P. Supercritical CO2-Ethanol Extraction of Oil from Green Coffee Beans: Optimization Conditions and Bioactive Compound Identification. J. Food Sci. Technol. 2021, 58, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Pinho, D.; Cardeal, Z.; Menezes, H.; Queiroz, M.; Pereira, O. Simplicillium Coffeanum, a New Endophytic Species from Brazilian Coffee Plants, Emitting Antimicrobial Volatiles. Phytotaxa 2018, 333, 188. [Google Scholar] [CrossRef]
- Juarez, G.F.Y.; Pabiloña, K.B.C.; Manlangit, K.B.L.; Go, A.W. Direct Dilute Acid Hydrolysis of Spent Coffee Grounds: A New Approach in Sugar and Lipid Recovery. Waste Biomass Valorization 2018, 9, 235–246. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical Fluid Extraction of Lipids from Spent Coffee Grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of Lipids from Spent Coffee Grounds Using Organic Solvents and Supercritical Carbon Dioxide. J. Food Process. Preserv. 2013, 37, 1014–1021. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Paula Robalo, M.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 Extraction of Spent Coffee Grounds. Influence of Co-Solvents and Characterization of the Extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Sharma, A.; Ray, A.; Singhal, R.S. A Biorefinery Approach towards Valorization of Spent Coffee Ground: Extraction of the Oil by Supercritical Carbon Dioxide and Utilizing the Defatted Spent in Formulating Functional Cookies. Future Foods 2021, 4, 100090. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced Extraction of Spent Coffee Grounds Oil Using High-Pressure CO2 plus Ethanol Solvents. Ind. Crops Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Matias, A.; Duarte, C.M.M.; Bronze, M.R. High-Pressure CO2 Assisted Extraction as a Tool to Increase Phenolic Content of Strawberry-Tree (Arbutus Unedo) Extracts. J. CO2 Util. 2018, 27, 73–80. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, e593902. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-Inflammatory Properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M.; Choi, M.-S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in Db/Db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Grujic, N.; Lepojevic, Z.; Srdjenovic, B.; Vladic, J.; Sudji, J. Effects of Different Extraction Methods and Conditions on the Phenolic Composition of Mate Tea Extracts. Molecules 2012, 17, 2518–2528. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An Outlook on Chlorogenic Acids—Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Cabral, F.A.; Meirelles, A.J.A. Ferulic Acid Solubility in Supercritical Carbon Dioxide, Ethanol and Water Mixtures. J. Chem. Thermodyn. 2016, 103, 285–291. [Google Scholar] [CrossRef]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Bergs, D.; Merz, J.; Delp, A.; Joehnck, M.; Martin, G.; Schembecker, G. A Standard Procedure for the Selection of Solvents for Natural Plant Extraction in the Early Stages of Process Development. Chem. Eng. Technol. 2013, 36, 1739–1748. [Google Scholar] [CrossRef]
- Knothe, G. Structure Indices in FA Chemistry. How Relevant Is the Iodine Value? J. Am. Oil Chem. Soc. 2002, 79, 847–854. [Google Scholar] [CrossRef]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Spent Coffee Grounds as a Renewable Source of Bioactive Compounds. J. Biobased Mater. Bioenergy 2013, 7, 420–428. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, N. Valorization of Spent Coffee Grounds as the Specialty Material for Dullness and Aging of Skin Treatments. Chem. Biol. Technol. Agric. 2021, 8, 55. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Akgün, N.A.; Bulut, H.; Kikic, I.; Solinas, D. Extraction Behavior of Lipids Obtained from Spent Coffee Grounds Using Supercritical Carbon Dioxide. Chem. Eng. Technol. 2014, 37, 1975–1981. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Palafox-Rivera, P.; Lugo-Flores, M.A.; Gaitán-Hernández, R.; González-Aguilar, G.A.; Silva-Espinoza, B.A.; Tortoledo-Ortiz, O.; Ayala-Zavala, J.F.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Contribution of Bioactive Compounds to the Antioxidant Capacity of the Edible Mushroom Neolentinus Lepideus. Chem. Biodivers. 2021, 18, e2100085. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans—A Review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef]
- De Toledo, P.R.A.B.; de Melo, M.M.R.; Rodrigues, V.H.; Pezza, H.R.; Rocha, S.M.; Toci, A.T.; Pezza, L.; Portugal, I.; Silva, C.M. Design of Volatile Organic Compounds Profiles of Roasted Coffea Arabica Extracts Produced by Supercritical and Conventional Solvents. Int. J. Food Sci. Technol. 2022, 57, 5479–5493. [Google Scholar] [CrossRef]
- Vatansever, S.; Hall, C. Flavor Modification of Yellow Pea Flour Using Supercritical Carbon Dioxide + Ethanol Extraction and Response Surface Methodology. J. Supercrit. Fluids 2020, 156, 104659. [Google Scholar] [CrossRef]
- Poisson, L.; Schmalzried, F.; Davidek, T.; Blank, I.; Kerler, J. Study on the Role of Precursors in Coffee Flavor Formation Using In-Bean Experiments. J. Agric. Food Chem. 2009, 57, 9923–9931. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Role of Roasting Conditions in the Profile of Volatile Flavor Chemicals Formed from Coffee Beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of Coffee Flavor: A Compositional and Sensory Perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, D.; del Pilar Sánchez-Camargo, A. Study of the Fatty Acid Profile and the Aroma Composition of Oil Obtained from Roasted Colombian Coffee Beans by Supercritical Fluid Extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
| Code | Extraction Methods | Time (h) | % Ethanol (Cosolvent) | Yield (g/100 g d.m.) |
|---|---|---|---|---|
| Ce | Ethanol | 5 | 0 | 15.95 ± 0.11 a |
| Cs | n-Hexane | 5 | 0 | 16.35 ± 1.71 a |
| SC0 | Supercritical CO2 | 1 | 0 | 14.21 ± 0.15 b |
| SC5 | Supercritical CO2 | 1 | 5 | 15.45 ± 0.09 ab |
| L0 | Liquid CO2 | 1 | 0 | 14.48 ± 0.07 b |
| L5 | Liquid CO2 | 1 | 5 | 15.88 ± 0.18 a |
| Ce | Cs | SC0 | SC5 | L0 | L5 | |
|---|---|---|---|---|---|---|
| TPC (mgGAE/100 g oil) | 1312.00 ± 19.00 a | 73.00 ± 1.53 d | 692.75 ± 55.00 bc | 857.25 ± 37.00 b | 419.50 ± 66.00 c | 969.75 ± 35.00 b |
| Phenolic acids (mg/100 g oil): | ||||||
| 3,4-Dihydroxybenzoic acid | 5.08 ± 0.46 b | n.d. | n.d. | 4.70 ± 0.11 b | n.d. | 9.08 ± 0.39 a |
| Caffeic acid | 1.04 ± 0.05 b | n.d. | n.d. | 3.16 ± 0.04 a | n.d. | 3.04 ± 0.87 a |
| Chlorogenic acid | 2.62 ± 0.01 a | n.d. | n.d. | 1.41 ± 0.16 c | n.d. | 2.01 ± 0.06 b |
| Ferulic acid | 2.47 ± 0.02 a | n.d. | n.d. | 1.96 ± 0.03 b | n.d. | 1.89 ± 0.12 b |
| Caffeine | 378.87 ± 30.98 b | 15.07 ± 0.77 e | 230.44 ± 31.23 cd | 579.21 ± 59.15 a | 120.58 ± 1.82 de | 276.75 ± 2.35 bc |
| Fatty Acid (%) | Ce | Cs | SC0 | SC5 | L0 | L5 |
|---|---|---|---|---|---|---|
| Palmitic acid (C16:0) | 32.21 ± 0.51 | 30.51 ± 0.19 | 31.38 ± 0.06 | 27.84 ± 3.52 | 30.52 ± 0.35 | 31.06 ± 1.18 |
| Stearic acid (C18:0) | 7.47 ± 0.05 | 7.77 ± 0.08 | 7.47 ± 0.09 | 8.21 ± 0.55 | 7.65 ± 0.08 | 7.96 ± 0.29 |
| Oleic acid (C18:1) | 9.70 ± 0.23 | 10.17 ± 0.29 | 9.58 ± 0.28 | 10.24 ± 0.41 | 10.09 ± 0.24 | 10.22 ± 0.01 |
| Linoleic acid (C18:2) | 46.14 ± 0.07 | 46.01 ± 0.21 | 46.36 ± 0.06 | 48.97 ± 3.68 | 46.17 ± 0.25 | 45.18 ± 0.87 |
| Arachidic acid (C20:0) | 2.08 ± 0.34 | 2.48 ± 0.10 | 2.35 ± 0.17 | 2.01 ± 0.64 | 2.42 ± 0.13 | 2.51 ± 0.13 |
| Linolenic acid (C18:3) | 1.21 ± 0.13 | 1.30 ± 0.03 | 1.23 ± 0.05 | 1.16 ± 0.18 | 1.32 ± 0.04 | 1.23 ± 0.19 |
| Cis-8,11,14 Eicosatrienoic acid (C20:3) | 0.75 ± 0.24 | 1.09 ± 0.04 | 1.03 ± 0.13 | 0.99 ± 0.20 | 1.05 ± 0.06 | 1.18 ± 0.08 |
| Σ SFA | 41.99 ± 0.09 | 41.11 ± 0.01 | 41.54 ± 0.18 | 38.36 ± 3.63 | 41.01 ± 0.15 | 41.91 ± 1.33 |
| Σ MUFAs | 9.90 ± 0.21 | 10.46 ± 0.29 | 9.82 ± 0.32 | 10.47 ± 0.34 | 10.41 ± 0.30 | 10.45 ± 0.17 |
| Σ PUFAs | 48.11 ± 0.30 | 48.43 ± 0.31 | 48.64 ± 0.14 | 51.17 ± 3.28 | 48.58 ± 0.15 | 47.64 ± 1.17 |
| ω-6/ω-3 | 39.00 ± 4.19 | 36.24 ± 0.89 | 38.63 ± 2.04 | 44.11 ± 10.58 | 35.60 ± 1.12 | 38.28 ± 5.12 |
| Ce | Cs | SC0 | SC5 | L0 | L5 | |
|---|---|---|---|---|---|---|
| DPPH | 3492.30 ± 28.10 a | 371.85 ± 29.43 b | 413.40 ± 70.52 b | 3088.50 ± 11.55 a | 370.50 ± 47.81 b | 3135.90 ± 54.71 a |
| FRAP | 4408.10 ± 14.23 a | 2758.10 ± 21.09 c | 3400.80 ± 21.45 b | 4382.60 ± 55.34 a | 2557.30 ± 64.22 c | 4323.60 ± 38.72 a |
| ABTS | 8351.00 ± 14.35 a | 890.99 ± 89.10 d | 3048.10 ± 15.32 c | 5508.30 ± 11.22 b | 5648.00 ± 11.04 b | 5396.30 ± 30.05 b |
| Ce | Cs | SC0 | SC5 | L0 | L5 | |
|---|---|---|---|---|---|---|
| Ʃ Furans | 60.08 b | 25.12 e | 37.50 d | 66.85 a | 61.11 ab | 45.15 c |
| Furfurale | 1.00 ± 0.13 d | 9.72 ± 0.63 a | n.d. | 4.32 ± 0.13 c | n.d. | 6.93 ± 0.35 b |
| 2-Furanmethanol | 51.01 ± 0.75 a | 1.91 ± 0.31 c | 30.45 ± 2.11 b | 54.46 ± 0.54 a | 51.12 ± 0.08 a | 33.88 ± 2.36 b |
| 2,2′-methylenebis-furan | 2.43 ± 0.07 c | 4.63 ± 0.05 a | 2.41 ± 0.08 b | 2.43 ± 0.25 bc | 3.24 ± 0.29 a | 1.40 ± 0.01 c |
| 2-Furanmethanol acetate | 3.59 ± 0.16 cd | 5.97 ± 0.49 ab | 2.39 ± 0.50 cd | 4.15 ± 0.25 bc | 6.75 ± 0.83 a | 2.03 ± 0.08 d |
| 2-(2-furanylmethyl)-5-methyl-furan | 2.05 ± 0.15 bc | 2.89 0.18 a | 2.25 ± 0.12 b | 1.49 ± 0.25 cd | n.d. | 0.91 ± 0.04 d |
| Ʃ Lactone | 11.07 a | 11.94 a | 3.32 c | 14.39 a | 6.93 b | 7.05 b |
| Butyrolactone | 11.07 ± 0.19 a | 11.94 ± 0.06 a | 3.32 ± 0.30 c | 14.39 ± 1.94 a | 6.93 ± 0.75 b | 7.05 ± 0.29 b |
| Ʃ Phenols | 15.17 d | 32.58 b | 39.22 a | 9.03 e | 13.38 d | 19.56 c |
| Phenol | 2.78 ± 0.32 b | 3.75 ± 0.03 b | 6.08 ± 0.24 a | 1.20 ± 0.03 c | 3.95 ± 0.85 b | 5.60 ± 0.16 a |
| 2-methoxyphenol | 5.24 ± 0.18 b | 7.41 ± 0.79 a | 8.77 ± 1.06 a | 2.87 ± 0.12 c | 2.17 ± 0.05 c | 3.28 ± 0.16 c |
| 4-ethyl-2-methoxyphenol | 6.46 ± 0.01 bc | 21.42 ± 1.81 a | 19.90 ± 0.39 a | 3.90 ± 0.68 c | 5.01 ± 0.55 c | 9.76 ± 0.04 b |
| 2-methoxy-4-vinylphenol | 0.69 ± 0.02 cd | n.d. | 4.47 ± 0.48 a | 1.06 ± 0.15 c | 2.16 ± 0.11 b | 0.94 ± 0.12 c |
| Ʃ Pyrazines | 5.66 d | 12.20 b | 5.94 d | 5.09 d | 7.59 c | 15.43 a |
| 2-ethyl-6-methyl-pyrazine | 1.58 ± 0.33 d | 8.01 ± 0.56 b | 2.76 ± 0.11 c | 2.27 ± 0.22 cd | 2.40 ± 0.12 cd | 13.18 ± 1.23 a |
| 2-ethyl-3,5-dimethyl-pyrazine | 4.08 ± 0.16 ab | 4.19 ± 0.72 a | 3.18 ± 0.02 bc | 2.82 ± 0.32 bc | 5.19 ± 0.38 a | 2.24 ± 0.08 c |
| Ʃ Pyrroles | 8.04 d | 18.16 a | 14.03 b | 4.66 e | 11.08 c | 12.81 bc |
| 1H-pyrrole-2-carboxaldehyde | 0.97 ± 0.01 de | 2.49 ± 0.12 bc | 2.87 ± 0.38 b | 0.71 ± 0.13 e | 5.05 ± 0.32 a | 1.85 ± 0.28 cd |
| 5-methyl-1H-pyrrole-2-carboxaldehyde | 1.44 ± 0.01 a | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-(1H-pyrrol-2-yl)-ethanone | 2.57 ± 0.1 e | 12.68 ± 0.38 a | 6.22 ± 0.96 c | 1.58 ± 0.44 e | 4.10 ± 0.40 d | 9.10 ± 0.20 b |
| 1-(2-furanylmethyl)1H-pyrrole | 3.06 ± 0.21 ab | 2.99 ± 0.44 ab | 4.94 ± 0.81 a | 2.37 ± 0.31 b | 1.93 ± 0.40 b | 1.86 ± 0.36 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, R.; De Luca, L.; Basile, G.; Nitride, C.; Pizzolongo, F.; Masi, P. The Use of Carbon Dioxide as a Green Approach to Recover Bioactive Compounds from Spent Coffee Grounds. Foods 2023, 12, 1958. https://doi.org/10.3390/foods12101958
Romano R, De Luca L, Basile G, Nitride C, Pizzolongo F, Masi P. The Use of Carbon Dioxide as a Green Approach to Recover Bioactive Compounds from Spent Coffee Grounds. Foods. 2023; 12(10):1958. https://doi.org/10.3390/foods12101958
Chicago/Turabian StyleRomano, Raffaele, Lucia De Luca, Giulia Basile, Chiara Nitride, Fabiana Pizzolongo, and Paolo Masi. 2023. "The Use of Carbon Dioxide as a Green Approach to Recover Bioactive Compounds from Spent Coffee Grounds" Foods 12, no. 10: 1958. https://doi.org/10.3390/foods12101958