Pseudomonas fluorescens and Escherichia coli in Fresh Mozzarella Cheese: Effect of Cellobiose Oxidase on Microbiological Stability during Refrigerated Shelf Life
Abstract
:1. Introduction
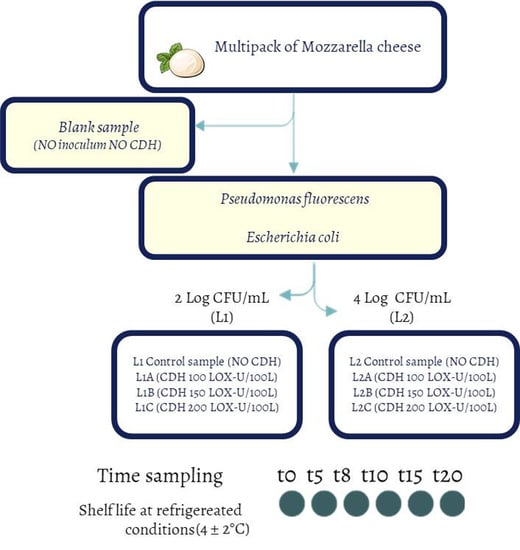
2. Materials and Methods
2.1. Samples
2.2. P. fluorescens and E. coli Strains
2.3. Microbiological Challenge Tests
2.4. Microbiological Analysis
2.5. Determination of Hydrogen Peroxide Concentration
3. Results
3.1. Evaluation of Endogenous Microbiota of Mozzarella Cheese
3.2. Effect of CDH toward P. fluorescens and E. coli in Mozzarella Cheese
3.2.1. Behavior of P. fluorescens in Mozzarella Cheese during Refrigerated Shelf Life
3.2.2. Behavior of E. coli in Mozzarella Cheese during Refrigerated Shelf Life
| L1 | L1 A | L1 B | L1 C | L2 | L2 A | L2 B | L2 C | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 1.92 | ± | 0.10 a | / | / | / | 3.81 | ± | 0.14 h | / | / | / | ||||||||||||
| T5 | >4 | 3.43 | ± | 0.66 b | 3.34 | ± | 0.56 b | 4.31 | ± | 0.66 b | > 6 | 4.53 | ± | 0.56 i | 4.47 | ± | 0.48 i | 4.30 | ± | 0.97 i | ||||
| T8 | 7.43 | ± | 0.15 cfg | 6.10 | ± | 0.29 cd | 6.04 | ± | 0.58 cd | 5.46 | ± | 0.42 | 7.44 | ± | 0.11 pq | 5.12 | ± | 0.36 lmn | 6.73 | ± | 0.33 mnop | 6.69 | ± | 0.02 lm |
| T10 | 7.48 | ± | 0.06 cfg | 5.45 | ± | 0.63 | 6.61 | ± | 0.22 de | 6.29 | ± | 0.32 de | 7.58 | ± | 0.11 q | 6.37 | ± | 0.40 lmnoq | 6.62 | ± | 0.03 nopq | 6.47 | ± | 0.35 mnopq |
| T15 | 7.43 | ± | 0.12 fg | 6.83 | ± | 0.17 def | 6.91 | ± | 0.32 ef | 6.90 | ± | 0.09 ef | 7.18 | ± | 0.11 pq | 7.40 | ± | 0.08 p | 7.04 | ± | 0.15 opq | 7.05 | ± | 0.15 op |
| T20 | 7.76 | ± | 0.09 g | 6.81 | ± | 0.23 def | 6.88 | ± | 0.10 ef | 6.87 | ± | 0.26 ef | 7.38 | ± | 0.14 q | 6.91 | ± | 0.19 opq | 7.00 | ± | 0.09 opq | 6.64 | ± | 0.13 opq |
3.3. Hydrogen Peroxide Concentrations in Mozzarella
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Angelis, M.; Gobetti, M. Stress Responses of Lactobacilli; Tsakalidou, E., Papadimitriou, K., Eds.; Springer: New York, NY, USA, 2011; pp. 219–249. [Google Scholar]
- Ah, J.; Tagalpallewar, G.P. Functional properties of mozzarella cheese for its end use application. J. Food Sci. Technol. 2017, 54, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Guidone, A.; Zotta, T.; Matera, A.; Ricciardi, A.; de Filippis, F.; Ercolini, D.; Parente, E. The microbiota of high-moisture mozzarella cheese produced with different acidification methods. Int. J. Food Microbiol. 2016, 216, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Angiolillo, L.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. The effect of incorporating calcium lactate in the saline solution on improving the shelf life of fiordilatte cheese. Int. J. Dairy Technol. 2013, 66, 373–381. [Google Scholar]
- Ottogalli, G.; Rondinini, G.; Conti, D. Coliformi, streptococchi fecali e test di sedimentazione su alcuni formaggi freschi. Ann. Microbiol. 1979, 29, 41–47. [Google Scholar]
- Massa, S.; Gardini, F.; Sinigaglia, M.; Guerzoni, M.E. Klebsiella pneumoniae as a spoilage organism in mozzarella cheese. J. Dairy Sci. 1992, 75, 1411–1414. [Google Scholar] [CrossRef]
- Altieri, C.; Manginelli, A.; Giudici, P. Evoluzione della carica microbica della mozzarella di latte vaccino in rapporto alla tecnologia di produzione. Il Latte 1994, 12, 1252–1256. [Google Scholar]
- Cantoni, C.; Iacumin, L.; Comi, G. Yellow-orange discolouration of mozzarella cheese. Ind. Aliment. 2003, 42, 134–136. [Google Scholar]
- Cantoni, C.; Stella, S.; Cozzi, M.; Iacumin, L.; Comi, G. Colorazione blu di mozzarelle. Ind. Aliment. 2003, 42, 840–843. [Google Scholar]
- Parisi, S. Evoluzione chimico-fisica e microbiologica nella conservazione di prodotti lattiero-caseari. Ind. Aliment. 2003, 42, 249–259. [Google Scholar]
- Parisi, S. Curve predittive per la crescita dei batteri Coliformi in prodotti lattiero-caseari. Ind. Aliment. 2003, 42, 29–37. [Google Scholar]
- Altieri, C.; Scrocco, C.; Sinigaglia, M.; del Nobile, M.A. Use of chitosan to prolong mozzarella cheese shelf life. J. Dairy Sci. 2005, 88, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Soncini, G.; Milesi, S.; Cocolin, L.; Iacumin, L. Colorazioni anomale e rigonfiamenti di formaggi fusi e mozzarelle. Ind. Aliment. 2006, 155, 276–281. [Google Scholar]
- Cantoni, C.; Bersani, C. Mozzarelle Blu: Cause Ed Ipotesi. Ind. Aliment. 2010, 40, 33–35. [Google Scholar]
- Cabrini, A.; Neviani, E. Il Genere Psesudomonas Causa di sapore amaro e di odore putrido sulla superficie di formaggio mozzarella. Il Latte 1983, 8, 90–93. [Google Scholar]
- Nanu, E.; Latha, C.; Sunil, B.; Prejit, M.T.; Menon, K.V. Quality assurance and public health safety of raw milk at the production point. Am. J. Food Technol. 2007, 2, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Zappia, A.; Branca, M.L.; Piscopo, A.; Poiana, M. Shelf life extension of mozzarella cheese packed in preserving liquid with calcium lactate and bergamot juice concentrate. J. Dairy Res. 2020, 87, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Eliot, S.C.; Vuillemard, J.C.; Emond, J.P. Stability of shredded mozzarella cheese under modified atmospheres. J. Food Sci. 1998, 63, 1075–1080. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Bevilacqua, A.; Corbo, M.R.; Pati, S.; Del Nobile, M.A. Use of active compounds for prolonging the shelf life of mozzarella cheese. Int. Dairy J. 2008, 18, 624–630. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Sinigaglia, M.; Del Nobile, M.A. Innovative active packaging systems to prolong the shelf life of mozzarella cheese. J. Dairy Sci. 2007, 90, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.J. Bioactive Components in Bovine Milk; Park, Y., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 13–42. [Google Scholar]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Monaci, L.; Deserio, D.; Morea, M.; Baruzzi, F. Antimicrobial efficacy of pepsin-digested bovine lactoferrin on spoilage bacteria contaminating traditional mozzarella cheese. Food Microbiol. 2012, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Gammariello, D.; Conte, A.; Attanasio, M.A. Combination of chitosan, coating and modified atmosphere packaging for prolonging fior di latte cheese shelf life. Carbohydr. Polym. 2009, 78, 151–156. [Google Scholar] [CrossRef]
- Laurienzo, P.; Malinconico, M.; Pizzano, R.; Manzo, C.; Piciocchi, N.; Sorrentino, A.; Volpe, M.G. Natural polysaccharide-based gels for dairy food preservation. J. Dairy Sci. 2006, 89, 2856–2864. [Google Scholar] [CrossRef] [Green Version]
- Sulej, J.; Osińska-Jaroszuk, M.; Jaszek, M.; Grąz, M.; Kutkowska, J.; Pawlik, A.; Chudzik, A.; Bancerz, R. Antimicrobial and antioxidative potential of free and immobilised cellobiose dehydrogenase isolated from wood degrading fungi. Fungal Biol. 2019, 123, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Harreither, W.; Sygmund, C.; Augustin, M.; Narciso, M.; Rabinovich, M.L.; Gorton, L.; Haltrich, D.; Ludwig, R. Catalytic properties and classification of cellobiose dehydrogenases from ascomycetes. Appl. Environ. Microbiol. 2011, 77, 1804–1815. [Google Scholar] [CrossRef] [Green Version]
- Zámocký, M.; Hallberg, M.; Ludwig, R.; Divne, C.; Haltrich, D. Ancestral gene fusion in cellobiose dehydrogenases reflects a specific evolution of gmc oxidoreductases in fungi. Gene 2004, 338, 1–14. [Google Scholar] [CrossRef]
- Bao, W.; Renganathan, V. Cellobiose oxidase of phanerochaete chrysosporium enhances crystalline cellulose degradation by cellulases. FEBS Lett. 1992, 302, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Henriksson, G.; Sild, V.; Szabó, I.J.; Pettersson, G.; Johansson, G. Substrate specificity of cellobiose dehydrogenase from Phanerochaete chrysosporium. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1998, 1383, 48–54. [Google Scholar] [CrossRef]
- Henriksson, G.; Johansson, G.; Pettersson, G. A critical review of cellobiose dehydrogenases. J. Biotechnol. 2000, 78, 93–113. [Google Scholar] [CrossRef]
- Sygmund, C.; Santner, P.; Krondorfer, I.; Peterbauer, C.K.; Alcalde, M.; Nyanhongo, G.S.; Guebitz, G.M.; Ludwig, R. Semi-rational engineering of cellobiose dehydrogenase for improved hydrogen peroxide production. Microb. Cell Fact. 2013, 12, 38. [Google Scholar] [CrossRef]
- Tegl, G.; Thallinger, B.; Beer, B.; Sygmund, C.; Ludwig, R.; Rollett, A.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial cellobiose dehydrogenase-chitosan particles. ACS Appl. Mater. Interfaces 2016, 8, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Nyanhongo, G.S.; Thallinger, B.; Guebitz, G.M. Cellobiose dehydrogenase-based biomedical applications. Process Biochem. 2017, 59, 37–45. [Google Scholar] [CrossRef]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of Action of Hydrogen Peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samoilenko, I.I.; Vasilyeva, E.I.; Pavlova, I.B.; Tumanian, M.A. Mechanisms of the bactericidal action of hydrogen peroxide. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1983, 60, 30–33. [Google Scholar]
- Rodrigues, R.d.S.; Machado, S.G.; de Carvalho, A.F.; Nero, L.A. Pseudomonas sp. as the Causative Agent of Anomalous Blue Discoloration in Brazilian Fresh Soft Cheese (Minas Frescal). Int. Dairy J. 2021, 117, 105020. [Google Scholar] [CrossRef]
- Miranda, J.M.; Franco, C.M.; Vázquez, B.I.; Fente, C.A.; Barros-Velázquez, J.; Cepeda, A. Evaluation of Chromocult® Enterococci Agar for the Isolation and Selective Enumeration of Enterococcus spp. in Broilers. Lett. Appl. Microbiol. 2005, 41, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Delbès, C.; Ali-Mandjee, L.; Montel, M.C. Monitoring Bacterial Communities in Raw Milk and Cheese by Culture-Dependent and -Independent 16S rRNA Gene-Based Analyses. Appl. Environ. Microbiol. 2007, 73, 1882–1891. [Google Scholar] [CrossRef] [Green Version]
- Faccia, M.; Gambacorta, G.; Natrella, G.; Caponio, F. Shelf Life Extension of Italian Mozzarella by Use of Calcium Lactate Buffered Brine. Food Control 2019, 100, 287–291. [Google Scholar] [CrossRef]
- Meier, F.; Lacroix, C.; Meile, L.; Jans, C. Enterococci and Pseudomonads as Quality Indicators in Industrial Production and Storage of Mozzarella Cheese from Raw Cow Milk. Int. Dairy J. 2018, 82, 28–34. [Google Scholar] [CrossRef]
- Thallinger, B.; Argirova, M.; Lesseva, M.; Ludwig, R.; Sygmund, C.; Schlick, A.; Nyanhongo, G.S.; Guebitz, G.M. Preventing Microbial Colonisation of Catheters: Antimicrobial and Antibiofilm Activities of Cellobiose Dehydrogenase. Int. J. Antimicrob. Agents 2014, 44, 402–408. [Google Scholar] [CrossRef]
- Parmar, Y.S.; Sharma, S.; Sharma, A.; Kaur, M. Extraction and Evaluation of Gibberellic Acid from Pseudomonas sp.: Plant Growth Promoting Rhizobacteria. J. Pharmacogn. Phytochem. 2018, 7, 2790–2795. [Google Scholar]
- Mladenović, K.G.; Muruzović, M.; Žugić Petrović, T.; Stefanović, O.D.; Čomić, L.R. Isolation and Identification of Enterobacteriaceae from Traditional Serbian Cheese and Their Physiological Characteristics. J. Food Saf. 2018, 38, e12387. [Google Scholar] [CrossRef]
- Elkins, J.G.; Hassett, D.J.; Stewart, P.S.; Schweizer, H.P.; McDermott, T.R. Protective Role of Catalase in Pseudomonas Aeruginosa Biofilm Resistance to Hydrogen Peroxide. Appl. Environ. Microbiol. 1999, 65, 4594–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, K. Effect of Hydrogen Peroxide on Antibacterial Activities of Canadian Honeys. Can. J. Microbiol. 2011, 52, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef]


| L1 | L1 A | L1 B | L1 C | L2 | L2 A | L2 B | L2 C | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 2.51 | ± | 0.06 a | / | / | / | 3.65 | ± | 0.17 fg | / | / | / | ||||||||||||
| T5 | 6.03 | ± | 0.31 b | 1.22 | ± | 0.52 cde | 1.11 | ± | 0.27 cde | <1 | 6.59 | ± | 0.18 n | 3.64 | ± | 0.37 fghi | 3.45 | ± | 0.21 fghil | 3.50 | ± | 0.17 fghil | ||
| T8 | 6.02 | ± | 0.35 b | 1.99 | ± | 0.56 ad | 1.95 | ± | 0.92 cde | 1.05 | ± | 0.14 de | 6.29 | ± | 0.26 n | 4.41 | ± | 0.47 fg | 3.45 | ± | 0.31 fghil | 3.38 | ± | 0.54 fghil |
| T10 | 6.14 | ± | 0.25 b | 2.44 | ± | 0.68 a | <1 | <1 | 6.67 | ± | 0.18 n | 4.79 | ± | 0.15 f | 2.39 | ± | 0.35 ghil | 2.76 | ± | 0.61 ghil | ||||
| T15 | 5.45 | ± | 0.31 b | 2.17 | ± | 0.61 cd | <1 | <1 | 5.11 | ± | 0.17 mn | 4.22 | ± | 0.33 fghi | 2.05 | ± | 0.14 hil | 1.91 | ± | 0.46 il | ||||
| T20 | 5.63 | ± | 0.33 b | <1 | <1 | <1 | 5.08 | ± | 0.34 mn | 3.17 | ± | 0.26 fghil | 2.18 | ± | 0.27 ghil | 1.07 | ± | 0.12 l | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrella, M.; Bertani, G.; Ricci, A.; Volpe, R.; Roustel, S.; Ferriani, F.; Nipoti, E.; Neviani, E.; Lazzi, C.; Bernini, V. Pseudomonas fluorescens and Escherichia coli in Fresh Mozzarella Cheese: Effect of Cellobiose Oxidase on Microbiological Stability during Refrigerated Shelf Life. Foods 2023, 12, 145. https://doi.org/10.3390/foods12010145
Marrella M, Bertani G, Ricci A, Volpe R, Roustel S, Ferriani F, Nipoti E, Neviani E, Lazzi C, Bernini V. Pseudomonas fluorescens and Escherichia coli in Fresh Mozzarella Cheese: Effect of Cellobiose Oxidase on Microbiological Stability during Refrigerated Shelf Life. Foods. 2023; 12(1):145. https://doi.org/10.3390/foods12010145
Chicago/Turabian StyleMarrella, Martina, Gaia Bertani, Annalisa Ricci, Rossana Volpe, Sebastien Roustel, Federico Ferriani, Elia Nipoti, Erasmo Neviani, Camilla Lazzi, and Valentina Bernini. 2023. "Pseudomonas fluorescens and Escherichia coli in Fresh Mozzarella Cheese: Effect of Cellobiose Oxidase on Microbiological Stability during Refrigerated Shelf Life" Foods 12, no. 1: 145. https://doi.org/10.3390/foods12010145