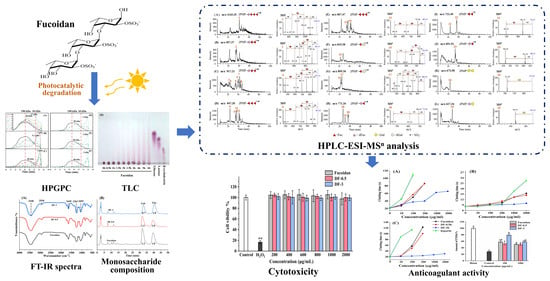
Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photocatalytic Degradation of Fucoidan
2.3. Chemical Analysis
2.4. Determination of Molecular Weight Distribution
2.5. Thin Layer Chromatography (TLC)
2.6. Analysis of Monosaccharide Composition
2.7. Fourier-Transform Infrared (FT-IR) Spectroscopic Analysis
2.8. Mass Spectrometry Analysis
2.9. Anticoagulant Activity
2.10. Cell Culture Assay
2.11. Cytotoxicity Study in HT-29 Cells
2.12. Chromogenic Factor XII Activation Assay
2.13. Statistical Analysis
3. Results and Discussions
3.1. Optimization of Photocatalytic Degradation Conditions
3.2. Chemical Composition Analysis
3.3. Identification of Oligosaccharides Produced by Photocatalytic Degradation
3.4. Cytotoxicity of the Degraded Fucoidans
3.5. Anticoagulant Activity of the Degraded Fucoidans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul. Fibrinolysis 2009, 20, 607–610. [Google Scholar] [CrossRef]
- Atashrazm, F. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.Y.; Shin, Y.E.; Kim, H.K. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res. 2019, 65, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel Antiviral Fucoidan from Sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhaohui, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Q.; Chen, L.; Ren, S.; Xu, P.; Tang, Y.; Luo, D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb. Res. 2010, 125, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ku, S.K.; Kim, H.J.; Han, J.S. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem. Toxicol. 2012, 50, 4468–4478. [Google Scholar] [CrossRef] [PubMed]
- Pielesz, A.; Biniaś, W.; Paluch, J. Mild acid hydrolysis of fucoidan: Characterization by electrophoresis and FT-Raman spectroscopy. Carbohydr. Res. 2011, 346, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Koo, Y.K.; Jung, M.K.; Moon, H.R.; Kim, S.M.; Synytsya, A.; Yun-Choi, H.S.; Kim, Y.S.; Park, J.K.; Park, Y.I. Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Arch. Pharmacal Res. 2010, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ye, X.; Sun, Y.; Wu, D.; Wu, N.; Hu, Y.; Chen, S. Ultrasound Effects on the Degradation Kinetics, Structure, and Antioxidant Activity of Sea Cucumber Fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, A.; Singh, S.K. Acid hydrolysis of sulphated polysaccharides. Desulphation and the effect on molecular mass. Carbohydr. Polym. 1999, 38, 7–15. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X. Ultrasonic degradation effects on the physicochemical, rheological and antioxidant properties of polysaccharide from Sargassum pallidum. Carbohydr. Polym. 2020, 239, 116230. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; Chen, W.Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Zhao, J.; Li, D.M.; Song, S.; Song, L.; Fu, Y.H.; Zhang, L.P. Structural investigation of a uronic acid-containing polysaccharide from abalone by graded acid hydrolysis followed by PMP-HPLC–MSn and NMR analysis. Carbohydr. Res. 2015, 402, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.-C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm: Sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Wong, C.C. The photocatalytic degradation of dicamba in TiO2 suspensions with the help of hydrogen peroxide by different near UV irradiations. Water Res. 2004, 38, 1037–1043. [Google Scholar] [CrossRef]
- Miller, A.R. Oxidation of cell wall polysaccharides by hydrogen peroxide: A potential mechanism for cell wall breakdown in plants. Biochem. Biophys. Res. Commun. 1986, 141, 238–244. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Carbajo, J.; Bahamonde, A.; Martínez-Arias, A. Phenol photodegradation with oxygen and hydrogen peroxide over TiO2 and Fe-doped TiO2. Catal. Today 2009, 143, 247–252. [Google Scholar] [CrossRef]
- Garcia, J.C.; Oliveira, J.L.; Silva, A.E.C.; Oliveira, C.C.; Nozaki, J.; de Souza, N.E. Comparative study of the degradation of real textile effluents by photocatalytic reactions involving UV/TiO2/H2O2 and UV/Fe2+/H2O2 systems. J. Hazard. Mater. 2007, 147, 105–110. [Google Scholar] [CrossRef]
- Lu, J.; Ai, C.; Guo, L.; Fu, Y.; Cao, C.; Song, S. Characteristic oligosaccharides released from acid hydrolysis for the structural analysis of chondroitin sulfate. Carbohydr. Res. 2017, 449, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Kwon, M.J.; Nam, T.J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017, 15, 4116–4122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Xu, L.; Cao, J.; Chen, W. Structural characterization of a broccoli polysaccharide and evaluation of anti-cancer cell proliferation effects. Carbohydr. Polym. 2015, 126, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Mao, W.J.; Fang, F.; Li, H.Y.; Sun, H.H.; Chen, Y.; Qi, X.H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008, 71, 428–434. [Google Scholar] [CrossRef]
- Wilbs, J.; Kong, X.D.; Middendorp, S.J.; Prince, R.; Cooke, A.; Demarest, C.T.; Abdelhafez, M.M.; Roberts, K.; Umei, N.; Gonschorek, P.; et al. Cyclic peptide FXII inhibitor provides safe anticoagulation in a thrombosis model and in artificial lungs. Nat. Commun. 2020, 11, 3890. [Google Scholar] [CrossRef] [PubMed]







| Sample | Average Mw/kDa | Total Sugar % | Sulfate Group % | Sulfated Polysaccharides % |
|---|---|---|---|---|
| Fucoidan | 190 | 47.52 ± 1.93 | 26.22 ± 2.04 | 97.10 ± 0.01 |
| DF-0.5 | 90 | 48.46 ± 1.00 | 23.08 ± 0.92 | 95.85 ± 0.04 |
| DF-3 | 3 | 36.46 ± 2.49 ** | 22.69 ± 0.72 | 57.70 ± 0.01 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, L.; You, Y.; Sun, X.; Wen, C.; Fu, Y.; Song, S. Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method. Foods 2022, 11, 822. https://doi.org/10.3390/foods11060822
Qi Y, Wang L, You Y, Sun X, Wen C, Fu Y, Song S. Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method. Foods. 2022; 11(6):822. https://doi.org/10.3390/foods11060822
Chicago/Turabian StyleQi, Yihui, Lilong Wang, Ying You, Xiaona Sun, Chengrong Wen, Yinghuan Fu, and Shuang Song. 2022. "Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method" Foods 11, no. 6: 822. https://doi.org/10.3390/foods11060822