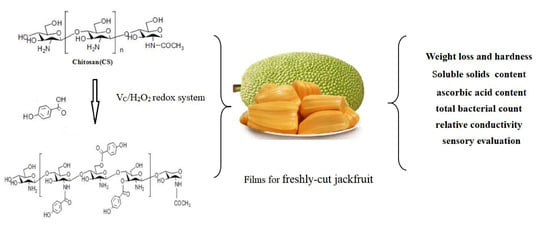
Functional Properties and Preservative Effect of P-Hydroxybenzoic Acid Grafted Chitosan Films on Fresh-Cut Jackfruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of PA-Grafted Chitosan
2.3. PA-g-CS Film Preparation
2.4. Characterization of PA-g-CS Films
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)Analysis
2.4.2. Scanning Electron Microscopy (SEM) of Films
2.4.3. Light Transmittance
2.4.4. Moisture Content (MC)
2.4.5. Water Solubility (WS)
2.4.6. Water Vapor Permeability (WVP)
2.4.7. Oxygen Permeability (OP)
2.4.8. Mechanical Properties of the Film
2.4.9. DPPH Radical Scavenging Activity
2.4.10. Antibacterial Activity
2.5. The Effect of CS and PA-g-CS Film on the Quality of Fresh-Cut Jackfruit
2.5.1. Jackfruit Preservation Treatment
2.5.2. Measurement of the Weight Loss in the Fruit
2.5.3. Hardness of the Fruit
2.5.4. Total Soluble Solids (TSS)
2.5.5. Estimation of Ascorbic Acid
2.5.6. Measurement of the Total Bacterial Count (TBC)
2.5.7. Membrane Permeability
2.5.8. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. The Properties of Films
3.1.1. FT-IR Spectra
3.1.2. Film Microstructure
3.1.3. Light Transmission
3.1.4. Moisture Content (MC) and Water Solubility (WS)
3.1.5. Water Vapor Permeability (WVP)
3.1.6. Oxygen Permeability (OP)
3.1.7. Tensile Strength (TS) and Elongation at Break (EB)
3.1.8. Antioxidant Activity of the Films
3.1.9. Antibacterial Activity
3.2. The Preservation Effect on Fresh-Cut Jackfruit
3.2.1. Weight Loss and Hardness
3.2.2. The Soluble Solids (TSS) and Ascorbic Acid Content
3.2.3. Total Bacterial Count
3.2.4. Membrane Relative Conductivity
3.2.5. The Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): A review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Xu, S.Y.; Liu, J.P.; Huang, X.S.; Du, L.P.; Shi, F.L.; Dong, R.; Huang, X.-T.; Zheng, K.; Liu, Y.; Cheong, K.-L.; et al. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. Lwt-Food Sci. Technol. 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Curutchet, A.; Dellacassa, E.; Ringuelet, J.A.; Chaves, A.R.; Viña, S.Z. Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha×piperita and M. spicata). Food Chem. 2014, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.; O’Beirne, D. Characterising deterioration patterns in fresh-cut fruit using principal component analysis. II: Effects of ripeness stage, seasonality, processing and packaging. Postharvest Biol. Technol. 2015, 100, 91–98. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.B.; Chung, H.S.; Moon, K.D. Browning control of fresh-cut lettuce by phytoncide treatment. Food Chem. 2014, 159, 188–192. [Google Scholar] [CrossRef]
- Mantilla, N.; Castell-Perez, M.E.; Gomes, C.; Moreira, R.G. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT-Food Sci. Technol. 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, T.L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 2016, 33, 580–588. [Google Scholar] [CrossRef]
- Wang, H.X.; Qan, J.; Ding, F.Y. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A.J.C.R. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Tonglairoum, P. 6-Maleimidohexanoic acid-grafted chitosan: A new generation mucoadhesive polymer. Carbohydr. Polym. 2018, 202, 258–264. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Deepak Kumar, R.; Rana, N.K.; Koch, B. Development of a novel chitosan based biocompatible and self-healing hydrogel for controlled release of hydrophilic drug. Int. J. Biol. Macromol. 2018, 116, 37–44. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Liu, J.; Meng Cg Liu, S.; Kan, J.; Jin, C.H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Yin, D.; Zeng, X. A comparative study on chitosan/gelatin composite films with conjugated or incorporated gallic acid. Carbohydr. Polym. 2017, 173, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural properties of films and rheology of film-forming solutions of chitosan gallate for food packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.J.; Kannan, K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ. Int. 2020, 136, 105465. [Google Scholar] [CrossRef] [PubMed]
- Castada, H.Z.; Sun, Z.; Barringer, S.A.; Huang, X. Thermal degradation of p-hydroxybenzoic acid in macadamia nut oil, olive oil, and corn oil. J. Am. Oil Chem. Soc. 2020, 97, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Jiang, Z.G. Synthesis, characterization, antioxidant and antibacterial properties of p-hydroxybenzoic acid-grafted chitosan conjugates. Int. J. Food Sci. Technol. 2022, 57, 1283–1290. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Ge, H.; Chen, L.; Wang, J.; Zhang, S.; Guo, Y.; Li, Z.; Feng, X. Physical properties and antioxidant capacity of chitosan/epigallocatechin-3-gallate films reinforced with nano-bacterial cellulose. Carbohydr. Polym. 2018, 179, 207–220. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Benjakul, S.; Prodpran, T.; Nilsuwan, K.; Huda, N.; Caba, K.D.L. Composite films based on chitosan and epigallocatechin gallate grafted chitosan: Characterization, antioxidant and antimicrobial activities. Food Hydrocoll. 2021, 111, 106384. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, X.; Chen, J.Y.; Gan, Z.Y.; Wan, C.P. Mitigating effects of chitosan coating on postharvest senescence and energy depletion of harvested pummelo fruit response to granulation stress. Food Chem. 2021, 348, 129113. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Sun, T.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Li, L.; Shao, Z. Functional properties and preservative effect on Penaeus vannamei of chitosan films with conjugated or incorporated chlorogenic acid. Int. J. Biol. Macromol. 2020, 159, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the physical properties and biological activity of chitosan films grafted with gallic acid and caffeic acid: A comparison study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Aljawish, A.; Muniglia, L.; Klouj, A.; Jasniewski, J.; Scher, J.; Desobry, S. Characterization of films based on enzymatically modified chitosan derivatives with phenol compounds. Food Hydrocoll. 2016, 60, 551–558. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Hu, F.; Sun, T.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Li, L.; Bian, X.; Shao, Z. Functional properties of chitosan films with conjugated or incorporated salicylic acid. J. Mol. Struct. 2021, 1223, 129237. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Schilling, M.W.; Mahmoud, B. Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical and barrier properties. Carbohydr. Polym. 2016, 151, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; García, M.A.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, H.; Shi, J.; Neethirajan, S.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef]
- Gao, P.; Zhu, Z.; Zhang, P. Effects of chitosan–glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, M.; Wang, S.; Cai, J.; Zhou, X.; Zhu, C. Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. LWT-Food Sci. Technol. 2010, 43, 519–524. [Google Scholar] [CrossRef]










| Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|
| CS Film | PA-g-CS-I Film | PA-g-CS-II Film | PA-g-CS-III Film | |
| E. coli | 11.3 ± 0.6 a | 13.9 ± 0.5 b | 16.3 ± 0.8 c | 29.0 ± 1.0 d |
| S. aureus | 13.4 ± 0.6 b | 15.2 ± 0.6 e | 18.7 ± 0.8 f | 22.2 ± 1.1 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wang, J.; Xiang, D.; Zhang, Z. Functional Properties and Preservative Effect of P-Hydroxybenzoic Acid Grafted Chitosan Films on Fresh-Cut Jackfruit. Foods 2022, 11, 1360. https://doi.org/10.3390/foods11091360
Jiang Z, Wang J, Xiang D, Zhang Z. Functional Properties and Preservative Effect of P-Hydroxybenzoic Acid Grafted Chitosan Films on Fresh-Cut Jackfruit. Foods. 2022; 11(9):1360. https://doi.org/10.3390/foods11091360
Chicago/Turabian StyleJiang, Zhiguo, Jiaolong Wang, Dong Xiang, and Zhengke Zhang. 2022. "Functional Properties and Preservative Effect of P-Hydroxybenzoic Acid Grafted Chitosan Films on Fresh-Cut Jackfruit" Foods 11, no. 9: 1360. https://doi.org/10.3390/foods11091360