Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production
Abstract
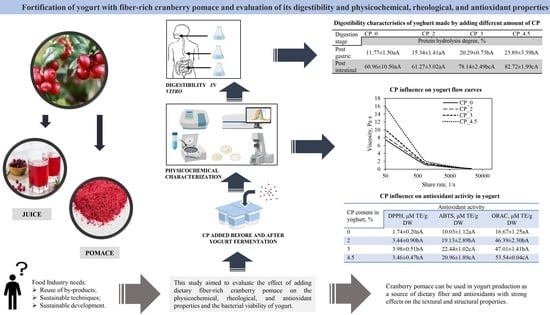
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Yogurt Fortified with Cranberry Pomace
2.3. Methods for Characterization of Yogurt with Cranberry Pomace
2.3.1. Chemical Composition of Cranberry Pomace and Yogurt with Cranberry Pomace
2.3.2. Syneresis
2.3.3. Particle Size and Distribution
2.3.4. Rheology Assessment
2.3.5. Scanning Electron Microscopy
2.3.6. Lactic Acid Bacteria (LAB) Viable Cell Counts
2.3.7. Total Phenol Content
2.3.8. Antioxidant Activity
DPPH Radical Scavenging Capacity
ABTS Radical Scavenging Activity
ORAC Analysis
2.4. Methods for Characterization of the Digestibility of Yogurt with Cranberry Pomace
2.4.1. In Vitro Digestion Model
2.4.2. Free α-Amino Groups by Fluorescamine Assay
2.4.3. The Bioaccessibility Index (BI) of the Phenol Compounds
2.5. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characterization of Yogurt Fortified with Fiber-Rich Cranberry Pomace
3.1.1. Chemical Composition of Cranberry Pomace and Yogurt Supplemented with Cranberry Pomace
3.1.2. Rheological and Structural Properties of Yogurt Fortified with Fiber-Rich Cranberry Pomace
3.1.3. Viability of Yogurt Culture Bacteria in the Yogurt Fortified with Fiber-Rich Cranberry Pomace
3.1.4. Antioxidant Activity of Yogurt Fortified with Fiber-Rich Cranberry Pomace
3.2. Digestibility of Yogurt Fortified with Fiber-Rich Cranberry Pomace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and Phenolic Acids from Cranberry Juice Are Bioavailable and Bioactive in Healthy Older Adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Ehret, D.; Godfrey, D.; Fukumoto, L.; Diarra, M. Characterization of Pilot Scale Processed Canadian Organic Cranberry (Vaccinium Macrocarpon) and Blueberry (Vaccinium Angustifolium) Juice Pressing Residues and Phenolic-Enriched Extractives. Int. J. Fruit Sci. 2017, 17, 202–232. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Simonsen, J.; Zhao, Y. Fruit Pomace as a Source of Alternative Fibers and Cellulose Nanofiber as Reinforcement Agent to Create Molded Pulp Packaging Boards. Compos. Part A Appl. Sci. Manuf. 2017, 99, 48–57. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of Valuable Lipophilic and Polyphenolic Fractions from Cranberry Pomace by Consecutive Supercritical CO2 and Pressurized Liquid Extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Islam, R.; Hassan, Y.I.; Das, Q.; Lepp, D.; Hernandez, M.; Godfrey, D.V.; Orban, S.; Ross, K.; Delaquis, P.; Diarra, M.S. Dietary Organic Cranberry Pomace Influences Multiple Blood Biochemical Parameters and Cecal Microbiota in Pasture-Raised Broiler Chickens. J. Funct. Foods 2020, 72, 104053. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Arvaj, L.; Strange, P.; Goodwin, M.; Barbut, S.; Balamurugan, S. Effect of Cranberry Pomace on the Physicochemical Properties and Inactivation of Salmonella during the Manufacture of Dry Fermented Sausages. Curr. Res. Food Sci. 2021, 4, 636–645. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Proximate and Polyphenolic Characterization of Cranberry Pomace. J. Agric. Food Chem. 2010, 58, 4030–4036. [Google Scholar] [CrossRef]
- Spadoni Andreani, E.; Karboune, S.; Liu, L. Extraction and Characterization of Cell Wall Polysaccharides from Cranberry (Vaccinium Macrocarpon Var. Stevens) Pomace. Carbohydr. Polym. 2021, 267, 118212. [Google Scholar] [CrossRef]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of Dietary Fiber on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R. In-Vitro Prebiotic Analysis of Microbiota Accessible Pectic Polysaccharides. Curr. Microbiol. 2019, 76, 1452–1460. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vattem, D.A.; Lin, Y.-T.; Labbe, R.G.; Shetty, K. Phenolic Antioxidant Mobilization in Cranberry Pomace by Solid-State Bioprocessing Using Food Grade Fungus Lentinus Edodes and Effect on Antimicrobial Activity against Select Food Borne Pathogens. Innov. Food Sci. Emerg. Technol. 2004, 1, 81–91. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Viticulture 1977, 28, 49–55. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ganske, F.; Dell, E.J. ORAC Assay on the FLUOstar OPTIMA to Determine Antioxidant Capacity; BMG LABTECH: Ortenberg, Germany, 2006. [Google Scholar]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORAC(FL))) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Jensen, H.B.; Sundekilde, U.K.; Clausen, M.R.; Eggers, N.; Larsen, L.B.; Ray, C.; Andersen, H.J.; Bertram, H.C. Chemical and Proteolysis-Derived Changes during Long-Term Storage of Lactose-Hydrolyzed Ultrahigh-Temperature (UHT) Milk. J. Agric. Food Chem. 2014, 62, 11270–11278. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Physicochemical Property Changes of Cabbage Outer Leaves upon Preparation into Functional Dietary Fiber Powder. Food Bioprod. Processing 2012, 90, 541–548. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- OJ L 404, 30.12.2006; Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Publications Office of the EU: Luxembourg, 2006; pp. 9–25.
- Xinya Wang; Eleana Kristo; Gisèle LaPointe Adding Apple Pomace as a Functional Ingredient in Stirred-Type Yogurt and Yogurt Drinks. Food Hydrocoll. 2020, 100, 105453. [CrossRef]
- Tseng, A.; Zhao, Y. Wine Grape Pomace as Antioxidant Dietary Fibre for Enhancing Nutritional Value and Improving Storability of Yogurt and Salad Dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lucey, J. Cultured Dairy Products: An Overview of Their Gelation and Texture Properties. Int. J. Dairy Technol. 2004, 57, 77–84. [Google Scholar] [CrossRef]
- Vianna, F.S.; Canto, A.C.V.C.S.; da Costa-Lima, B.R.C.; Salim, A.P.A.A.; Costa, M.P.; Balthazar, C.F.; Oliveira, B.R.; Rachid, R.P.; Franco, R.M.; Conte-Junior, C.A.; et al. Development of New Probiotic Yoghurt with a Mixture of Cow and Sheep Milk: Effects on Physicochemical, Textural and Sensory Analysis. Small Rumin. Res. 2017, 149, 154–162. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of Pectin-Rich Orange Fibre on Gel Characteristics and Sensory Properties in Lactic Acid Fermented Yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, Textural and Rheological Properties of Probiotic Yogurt Fortified with Fibre-Rich Pineapple Peel Powder during Refrigerated Storage. LWT Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- García-Pérez, F.J.; Sendra, E.; Lario, Y.; Fernández-López, J.; Sayas-Barberá, E.; Pérez-Álvarez, J. Rheology of Orange Fiber Enriched Yogurt. Milchwissenschaft 2006, 61, 55–59. [Google Scholar]
- Puvanenthiran, A.; Stevovitch-Rykner, C.; Mccann, T.; Day, L. Synergistic Effect of Milk Solids and Carrot Cell Wall Particles on the Rheology and Texture of Yoghurt Gels. Food Res. Int. 2014, 64, 701–708. [Google Scholar] [CrossRef]
- Maroziene, A. Interaction of Pectin and Casein Micelles. Food Hydrocoll. 2000, 14, 391–394. [Google Scholar] [CrossRef]
- Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.-F.; Lacroix, M. Antimicrobial Effect of Cranberry Juice and Extracts. Food Control 2011, 22, 1413–1418. [Google Scholar] [CrossRef]
- CXS 243-2003; Standard for Fermented Milks. FAO/WHO Codex Alimentarius Commision: Rome, Italy, 2003; Revised in 2008, 2010, 2018.
- Espírito-Santo, A.P.; Lagazzo, A.; Sousa, A.L.O.P.; Perego, P.; Converti, A.; Oliveira, M.N. Rheology, Spontaneous Whey Separation, Microstructure and Sensorial Characteristics of Probiotic Yoghurts Enriched with Passion Fruit Fiber. Food Res. Int. 2013, 50, 224–231. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of Olive Pomace Ingredients into Yoghurts as a Source of Fibre and Hydroxytyrosol: Antioxidant Activity and Stability throughout Gastrointestinal Digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Nihal Durmus; Esra Capanoglu; Meral Kilic-Akyilmaz Activity and Bioaccessibility of Antioxidants in Yoghurt Enriched with Black Mulberry as Affected by Fermentation and Stage of Fruit Addition. Int. Dairy J. 2021, 117, 105018. [CrossRef]
- Sabater, C.; Abad-García, C.; Delgado-Fernández, P.; Corzo, N.; Montilla, A. Carbohydrate Fraction Characterisation of Functional Yogurts Containing Pectin and Pectic Oligosaccharides through Convolutional Networks. J. Food Compos. Anal. 2020, 90, 103484. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent Developments on Polyphenol–Protein Interactions: Effects on Tea and Coffee Taste, Antioxidant Properties and the Digestive System. Food Funct. 2012, 3, 592. [Google Scholar] [CrossRef]
- Xiong, J.; Chan, Y.H.; Rathinasabapathy, T.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Enhanced Stability of Berry Pomace Polyphenols Delivered in Protein-Polyphenol Aggregate Particles to an in Vitro Gastrointestinal Digestion Model. Food Chem. 2020, 331, 127279. [Google Scholar] [CrossRef]
- Morell, P.; Fiszman, S.; Llorca, E.; Hernando, I. Designing Added-Protein Yogurts: Relationship between in vitro Digestion Behavior and Structure. Food Hydrocoll. 2017, 72, 27–34. [Google Scholar] [CrossRef]
- van Aken, G.A.; Bomhof, E.; Zoet, F.D.; Verbeek, M.; Oosterveld, A. Differences in in Vitro Gastric Behaviour between Homogenized Milk and Emulsions Stabilised by Tween 80, Whey Protein, or Whey Protein and Caseinate. Food Hydrocoll. 2011, 25, 781–788. [Google Scholar] [CrossRef]
- Clegg, M.E.; Ranawana, V.; Shafat, A.; Henry, C.J. Soups Increase Satiety through Delayed Gastric Emptying yet Increased Glycaemic Response. Eur. J. Clin. Nutr. 2013, 67, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Jennifer Borreani; Empar Llorca; Virginia Larrea; Isabel Hernando Adding Neutral or Anionic Hydrocolloids to Dairy Proteins under in vitro Gastric Digestion Conditions. Food Hydrocoll. 2016, 57, 169–177. [CrossRef]





| Sample | Fat | Protein | Fiber | Soluble Fiber | Insoluble Fiber | Total Solids |
|---|---|---|---|---|---|---|
| CP | 7.13 ± 0.39 | 7.6 ± 0.09 | 72.67 ± 1.55 | 12.74 ± 0.09 | 59.93 ± 1.46 | 96.71 ± 0.96 |
| Yogurt with CP | ||||||
| CP_0 | 0.00 | 5.68 | 0.00 | 0.00 | 0.00 | 13.84 |
| CP_2 | 0.20 | 5.83 | 1.45 | 0.25 | 1.20 | 15.66 |
| CP_3 | 0.29 | 5.90 | 2.18 | 0.38 | 1.80 | 16.56 |
| CP_4.5 | 0.44 | 6.01 | 3.27 | 0.57 | 2.70 | 17.92 |
| CP Content in Yogurt, % | Rheological Characteristics from Flow Curves and Mechanical Spectra 3 | Syneresis, % | Average Particle Size, D 4.3 (nm) | |||
|---|---|---|---|---|---|---|
| τ0 1, Pa | K 1 (Pa sn) | G′ 2, Pa | Tanδ 2 | |||
| 0 | 12.95 ± 0.30 a | 0.12 ± 0.00 a | 138 ± 18.08 a | 0.30 ± 0.00 a | 24 ± 2 a | 59.14 ± 0.86 a |
| I method | ||||||
| 2 | 19.53± 1.48 bA | 0.17 ± 0.00 bA | 225 ± 11.72 bcA | 0.31 ± 0.00 aA | 13 ± 3 bA | 58.35 ± 4.68 abA |
| 3 | 20.90 ± 3.07 bcA | 0.20 ± 0.00 cdA | 261 ± 5.29 cA | 0.33 ± 0.01 bA | 11 ± 2 cdA | 72.94 ± 3.80 bA |
| 4.5 | 24.32 ± 1.46 cA | 0.22 ± 0.02 dA | 348 ± 19.01 dA | 0.34 ± 0.00 bA | 9 ± 1 dA | 101.02 ± 10.83 cA |
| II method | ||||||
| 2 | 15.30 ± 0.10 bB | 0.17 ± 0.02 bA | 233 ± 17.39 bA | 0.33 ± 0.00 bB | 12 ± 2 bA | 67.94 ± 2.69 bA |
| 3 | 18.96 ± 0.96 cA | 0.18 ± 0.02 bcA | 283 ± 17.44 cdA | 0.34 ± 0.00 bcA | 7 ± 3 cB | 77.42 ± 2.94 cA |
| 4.5 | 36.16 ± 0.85 dB | 0.29 ± 0.00 cB | 419 ± 8.08 dB | 0.37 ± 0.01 cB | 6 ± 1 cB | 113.27 ± 7.47 dB |
| CP Content in Yogurt, % | Total Phenols Content, µg GAE/g Sample | Antioxidant Activity | ||
|---|---|---|---|---|
| DPPH, μM TE/g DW | ABTS, μM TE/g DW | ORAC, μM TE/g DW | ||
| 0 | 62.67 ± 2.73 aA | 1.74 ± 0.20 aA | 10.03 ± 1.12 aA | 16.67 ± 1.25 aA |
| I method | ||||
| 2 | 125.20 ± 2.84 bA | 3.44 ± 0.90 bA | 19.13 ± 2.89 bA | 46.39 ± 2.30 bA |
| 3 | 138.40 ± 2.57 cA | 3.98 ± 0.51 bA | 22.44 ± 1.02 cA | 47.01 ± 1.41 bA |
| 4.5 | 188.95 ± 1.84 dA | 3.46 ± 0.47 bA | 20.96 ± 1.89 cA | 53.54 ± 0.04 cA |
| II method | ||||
| 2 | 118.59 ± 0.14 bB | 3.57 ± 0.72 bA | 20.16 ± 0.72 bA | 24.78 ± 0.99 bB |
| 3 | 139.09 ± 3.39 cA | 3.74 ± 0.17 bA | 24.37 ± 1.36 cB | 34.36 ± 2.56 cB |
| 4.5 | 167.39 ± 0.42 dB | 4.01 ± 0.63 bA | 28.76 ± 1.26 dB | 36.02 ± 0.91 dB |
| Digestion Stage | I Method | II Method | |||||
|---|---|---|---|---|---|---|---|
| CP_0 | CP_2 | CP_3 | CP_4.5 | CP_2 | CP_3 | CP_4.5 | |
| Protein hydrolysis degree, % | |||||||
| Post gastric | 11.77 ± 1.50 aA | 15.34 ± 1.41 aA | 20.29 ± 0.73 bA | 23.89 ± 3.39 bA | 10.05 ± 1.73 abB | 8.42 ± 5.39 abA | 7.96 ± 0.05 bB |
| Post intestinal | 60.96 ± 10.50 aA | 61.27 ± 3.02 aA | 78.14 ± 2.49 bcA | 82.72 ± 1.99 cA | 17.82 ± 2.06 aB | 18.62 ± 1.66 bB | 26.08 ± 4.51 bB |
| BI of phenolic compounds, % | |||||||
| Post gastric | - | 79.35 ± 0.47 bA | 78.76 ± 0.18 bA | 82.14 ± 0.69 cA | 71.56 ± 0.19 aB | 74.05 ± 0.28 bB | 76.73 ± 3.14 bA |
| Post intestinal | - | 93.61 ± 0.65 bA | 93.59 ± 2.82 baA | 93.75 ± 3.00 baA | 85.64 ± 1.82 aA | 85.70 ± 0.33 aA | 92.42 ± 0.52 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varnaitė, L.; Keršienė, M.; Šipailienė, A.; Kazernavičiūtė, R.; Venskutonis, P.R.; Leskauskaitė, D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods 2022, 11, 758. https://doi.org/10.3390/foods11050758
Varnaitė L, Keršienė M, Šipailienė A, Kazernavičiūtė R, Venskutonis PR, Leskauskaitė D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods. 2022; 11(5):758. https://doi.org/10.3390/foods11050758
Chicago/Turabian StyleVarnaitė, Laurita, Milda Keršienė, Aušra Šipailienė, Rita Kazernavičiūtė, Petras Rimantas Venskutonis, and Daiva Leskauskaitė. 2022. "Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production" Foods 11, no. 5: 758. https://doi.org/10.3390/foods11050758