Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties
Abstract
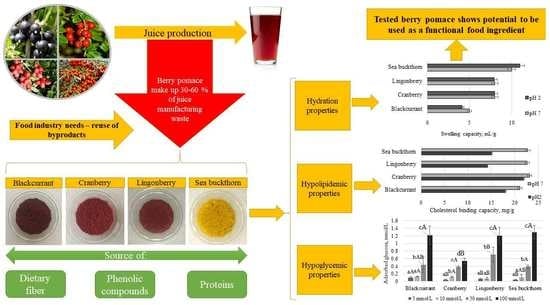
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Chemical Composition Analysis
2.3. Determination of the Techno-Functional Properties
2.3.1. Hydration Properties
2.3.2. Oil-Holding Capacity
2.4. Functional Properties
2.4.1. Glucose Adsorption Capacity
2.4.2. Glucose Diffusion
2.4.3. Cholesterol-Binding Capacity
2.4.4. Sodium-Cholate-Binding Capacity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Functional Properties of the Berry PP
3.2. In Vitro Hypoglycemic Effects
3.3. In Vitro Hypolipidemic Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low molecular weight phenolics of grape juice and winemaking byproducts: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and dna strand breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, Ö.; Hall, C.A., III. Fruit and Cereal Bioactives, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–8. [Google Scholar]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding value to fruit processing waste: Innovative ways to incorporate fibers from berry pomace in baked and extruded cereal-based foods—A SUSFOOD Project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.E.; Oomah, B.D.; Diarra, M.S.; Ibarra-Alvarado, C. Bioactivactivities of pilot-scale extracted cranberry juice and pomace. J. Food Process. Pres. 2013, 37, 356–365. [Google Scholar] [CrossRef]
- Ravi, H.K.; Breil, C.; Vian, M.A.; Chemat, F.; Venskutonis, P.R. Biorefining of Bilberry (Vaccinium Myrtillus L.) pomace using microwave hydrodiffusion and gravity, ultrasound-assisted, and bead-milling extraction. ACS Sustain. Chem. Eng. 2018, 6, 4185–4193. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Sablani, S.S.; Chiewchan, N.; Devahastin, S. Microwave-assisted extraction of sulforaphane from white cabbages: Effects of extraction condition, solvent and sample pretreatment. J. Food Eng. 2013, 117, 151–157. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace: Composition and technofunctional properties of berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Alba, K.; Campbell, G.M.; Kontogiorgos, V. Dietary fibre from berry processing waste and its impact on bread structure: A review. J. Sci. Food Agric. 2019, 99, 4189–4199. [Google Scholar] [CrossRef] [Green Version]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G. Evidence-based guideline of the german nutrition society: Carbohydrate intake and prevention of nutrition-related diseases. Ann. Nutr. Metab. 2012, 60, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Kabir, A.U.; Samad, M.B.; D’Costa, N.M.; Akhter, F.; Ahmed, A.; Hannan, J. Anti-HYPERGLYCEMIC Activity of Centella Asiatica Is Partly Mediated by Carbohydrase Inhibition and Glucose-Fiber Binding. BMC Complement. Altern. Med. 2014, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Alrington, VA, USA, 1995. [Google Scholar]
- Lee, S.C.; Prosky, L.; Vries, J.W.D. Determination of total, soluble, and insoluble dietary fiber in foods—Enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. AOAC Int. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Mcqueen, R.E.; Nicholson, J.W.G. Modification of the neutral-detergent fiber procedure for cereals and vegetables by using α-amylase. J. AOAC Int. 1979, 62, 676–680. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT Food Sci Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Wang, Q.; Zheng, B.; Lin, L.; Chen, B.; Zheng, Y.; Xiao, J. Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem. Toxicol. 2017, 109, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Bhutkar, M.A.; Bhinge, S.D.; Randive, D.S.; Wadkar, G.H. Hypoglycemic effects of berberis aristata and tamarindus indica extracts in vitro. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Huang, C.; Ou, S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J. Hazard. Mater. 2011, 186, 236–239. [Google Scholar] [CrossRef]
- Park, Y.W. Cholesterol contents of U.S. and imported goat milk cheeses as quantified by different colorimetric methods. Small Rumin. Res. 1999, 32, 77–82. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, Q.; Yuan, F.; Gao, Y. In vitro binding capacities and physicochemical properties of soluble fiber prepared by microfluidization pretreatment and cellulase hydrolysis of peach pomace. LWT Food Sci. Technol. 2015, 63, 677–684. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Sun, X.; Gong, W.; Ma, Y.; Liu, L.; Yao, J. Amino-Functionalized Cellulose: A Novel and High-Efficiency Scav-enger for Sodium Cholate Sorption. Cellulose 2020, 27, 4019–4028. [Google Scholar] [CrossRef]
- Spadoni Andreani, E.; Karboune, S. Comparison of Enzymatic and Microwave Assisted Alkaline Extraction Approaches for the Generation of Oligosaccharides from American Cranberry (Vaccinium macrocarpon) Pomace. J. Food Sci. 2020, 85, 2443–2451. [Google Scholar] [CrossRef]
- Alberici, N.; Fiorentini, C.; House, A.; Dordoni, R.; Bassani, A.; Spigno, G. Enzymatic Pre-Treatment of Fruit Pomace for Fibre Hydrolysis and Antioxidants Release. Chem. Eng. Trans. 2020, 79, 175–180. [Google Scholar] [CrossRef]
- Sójka, M.; Król, B. Composition of Industrial Seedless Black Currant Pomace. Eur. Food Res. Technol. 2009, 228, 597–605. [Google Scholar] [CrossRef]
- Ben-Mahmoud, Z.; Mohamed, M.S.; Bláha, J.; Lukešová, D.; Kunc, P. The Effect of Sea Buckthorn (Hippophae rhamnoides L.) Residues in Compound Feeds on the Performance and Skin Color of Broilers. Int. J. Anim. Res. 2014, 48, 548. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Corbu, A.R.; Ropota, M.; Turcu, R.P. Nutritional and Bioactive Compounds in Dried Sea-Buckthorn Pomace. Erwerbs-Obstbau 2021, 63, 91–98. [Google Scholar] [CrossRef]
- Hao, X.Y.; Ding, N.; Mu, C.T.; Zhang, C.X.; Zhao, J.X.; Zhang, J.X. Effects of Sea Buckthorn Pomace Supplementation on Energy Partitioning and Substrate Oxidation in Male Lambs. Anim. Feed Sci. Technol. 2019, 247, 149–156. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food Res. Int. 2021, 147, 110539. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Yang, G.; Sun, L.; Song, X.; Chen, Q.; Bao, Y.; Luo, T.; Wang, J. Microstructure, physicochemical properties, and adsorption capacity of deoiled red raspberry pomace and its total dietary fiber. LWT Food Sci. Technol. 2022, 153, 112478. [Google Scholar] [CrossRef]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT Food Sci. Technol. 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical characterization of five types of citrus dietary fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of Carrot (Daucus carota Linn. Var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Ma, Y.-S.; Tsai, Y.-H.; Chang, S.K.C. In vitro hypoglycemic, cholesterol-lowering and fermentation capacities of fiber-rich orange pomace as affected by extrusion. Int. J. Biol. Macromol. 2019, 124, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from Cocoa (Theobroma cacao L.) Shells. Food Funct. 2012, 3, 1044. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Sairam, S.; Urooj, A. In vitro hypoglycemic effects of selected dietary fiber sources. J. Food Sci. Technol. 2011, 48, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Céspedes, M.A.L.; Martínez Bustos, F.; Kil chang, Y. The effect of extruded orange pulp on enzymatic hydrolysis of starch and glucose retardation index. Food Bioproc. Technol. 2010, 3, 684–692. [Google Scholar] [CrossRef]
- Zhu, Y.; He, C.; Fan, H.; Lu, Z.; Lu, F.; Zhao, H. Modification of foxtail millet (Setaria Italica) bran dietary fiber by xylanase-catalyzed hydrolysis improves its cholesterol-binding capacity. LWT Food Sci. Technol. 2019, 101, 463–468. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jiménez, A.; Fernández-Bolaños, J.; Guillén, R.; Heredia, A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Tian, H.; Li, Y.; Shi, P.; Guo, W.; Zhu, Q. Effect of four modification methods on adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Res. Int. 2021, 147, 110565. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Wallace, R.G. Physico-chemical behavior of bile salts. Adv. Colloid Interface Sci. 1985, 22, 1–111. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X. Spectrometric study on the interaction of sodium cholate aggregates with quercetin. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 31–37. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine Coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Chu, J.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria Italic) bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]

| Parameters | Cranberry | Lingonberry | Sea Buckthorn | Black Currant |
|---|---|---|---|---|
| Moisture | 5.57 c ± 0.11 | 3.41 a ± 0.04 | 4.17 b ± 0.04 | 7.97 d ± 0.10 |
| Ash | 0.96 a ± 0.04 | 1.18 b ± 0.01 | 1.38 c ± 0.04 | 3.82 d ± 0.02 |
| Protein (N × 6.25) | 7.4 a ± 0.06 | 8.60 b ± 0.27 | 21.09 c ± 0.36 | 9.05 b ± 0.26 |
| Fat | 9.83 a ± 0.46 | 12.68 b ± 0.39 | 12.57 b ± 0.20 | 13.85 c ± 0.27 |
| Total dietary fiber | 72.67 c ± 1.55 | 73.85 c ± 0.83 | 63.61 b ± 1.64 | 49.24 a ± 0.95 |
| Total insoluble dietary fiber: | 59.93 b ± 1.46 | 65.36 c ± 0.67 | 58.69 b ± 0.96 | 40.95 a ± 0.78 |
| Cellulose | 17.14 a ± 2.01 | 17.89 a ± 1.88 | 13.62 b ± 2.24 | 7.45 c ± 1.04 |
| Acid-insoluble lignin | 39.58 a ± 1.55 | 42.08 b ± 1.73 | 39.23 a ± 2.09 | 30.16 c ± 1.91 |
| Total soluble dietary fiber | 12.74 c ± 0.09 | 8.49 b ± 0.05 | 4.92 a ± 0.68 | 8.29 b ± 0.17 |
| Soluble DF/insoluble DF ratio | 0.21 a | 0.13 b | 0.08 c | 0.20 a |
| Carbohydrates * | 9.14 a | 4.43 b | 1.35 c | 24.04 d |
| Total phenolic content, GAE/g DM | 3.89 a ± 0.29 | 6.26 b ± 0.23 | 5.73 c ± 0.02 | 11.06 d ± 0.40 |
| Powder of Pomace | Water Holding Capacity, (g/g) | Swelling Capacity, (mL/g) | Oil Binding Capacity, (g/g) | ||
|---|---|---|---|---|---|
| pH 2 | pH 7 | pH 2 | pH 7 | ||
| Black currant | 2.78 a ± 0.04 | 2.78 a ± 0.02 | 4.14 a ± 0.25 | 4.99 a ± 0.21 | 1.14 a ± 0.01 |
| Cranberry | 3.83 b ± 0.09 | 3.87 b ± 0.18 | 7.95 b ± 0.40 | 7.99 b ± 0.40 | 1.57 b ± 0.05 |
| Lingonberry | 3.28 c ± 0.05 | 3.27 c ± 0.02 | 7.90 b ± 0.40 | 8.00 b ± 0.40 | 1.46 c ± 0.05 |
| Sea buckthorn | 4.32 d ± 0.04 | 4.24 d ± 0.15 | 10.95 c ± 0.50 | 9.98 c ± 0.55 | 1.09 d ± 0.02 |
| Powder of Pomace | Glucose Concentration in the Dialysate, (mmol/g) | |||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| Control | 1.15 aA ± 0.01 | 1.58 bA ± 0.02 | 1.98 cA ± 0.03 | 2.10 cA ± 0.1 |
| Black currant | 0.96 aB ± 0.05 (16.61) * | 1.40 bB ± 0.07 (11.39) | 1.77 cB± 0.09 (10.96) | 1.92 cB ± 0.10 (8.46) |
| Cranberry | 1.03 aB ± 0.05 (10.40) | 1.48 bB ± 0.07 (6.40) | 1.86 cB ± 0.09 (5.97) | 2.05 cA ± 0.10 (2.22) |
| Lingonberry | 0.83 aC ± 0.04 (27.49) | 1.17 bC ± 0.06 (25.74) | 1.62 cC ± 0.08 (18.50) | 1.83 cB ± 0.09 (12.76) |
| Sea buckthorn | 1.00 aB ± 0.05 (13.30) | 1.48 bB ± 0.07 (6.13) | 1.93 cAB ± 0.10 (2.49) | 2.05 cA ± 0.10 (2.23) |
| Powder of Pomace | Cholesterol-Binding Capacity, mg/g | Sodium-Cholate-Binding Capacity, mg/g | |
|---|---|---|---|
| pH 2 | pH 7 | pH 7 | |
| Black currant | 18.02 a ± 0.01 | 21.11 a ± 0.42 | 74.78 a ± 1.39 |
| Cranberry | 21.91 b ± 0.02 | 23.13 b ± 0.47 | 52.68 b ± 2.07 |
| Lingonberry | 14.16 c ± 0.01 | 22.61 b ± 0.45 | 40.71 c ± 2.78 |
| Sea buckthorn | 15.11 d ± 0.06 | 22.75 b ± 0.46 | 24.66 d ± 5.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. https://doi.org/10.3390/foods11050716
Jurevičiūtė I, Keršienė M, Bašinskienė L, Leskauskaitė D, Jasutienė I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods. 2022; 11(5):716. https://doi.org/10.3390/foods11050716
Chicago/Turabian StyleJurevičiūtė, Ieva, Milda Keršienė, Loreta Bašinskienė, Daiva Leskauskaitė, and Ina Jasutienė. 2022. "Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties" Foods 11, no. 5: 716. https://doi.org/10.3390/foods11050716