The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
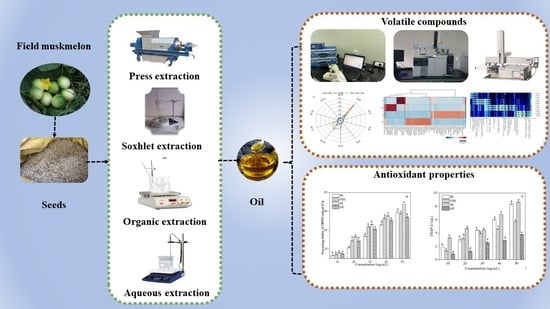
2.2. Extraction Method of Oil
2.3. Extraction Yield
2.4. Quality Indexes
2.5. Fatty Acid Composition
2.6. Comprehensive Analysis of Volatile Aroma Compounds of Seed Oil Samples
2.6.1. E-Nose
2.6.2. Headspace Solid Phase Microextraction Gas-Mass Spectrometry (HS-SPME-GC–MS)
2.6.3. Headspace Solid Phase Microextraction Ion Mobility Spectroscopy (HS-SPME-GC-IMS
2.7. Tocopherols
2.8. Antioxidant Activity
2.8.1. DPPH Radical Scavenging Ability
2.8.2. ABTS+ Radical Scavenging Activity
2.8.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. Data Analysis
3. Results and Discussion
3.1. Yield and Oil Quality Indexes
3.2. Fatty Acid Composition
3.3. E-nose Measures the Odor Profile
3.4. HS-SPME-GC-MS
3.5. HS-GS-IMS
3.5.1. Identification of Volatile Compounds in Seed Oil
3.5.2. Fingerprint Profile Comparisons in Seed Oil Samples by Different Extraction Methods
3.6. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, Z.; Ji, Y.; Xu, H.; Qiu, H.; Sun, L.; Zhong, H.; Liu, J. The potential contribution of growing rapeseed in winter fallow fields across Yangtze River Basin to energy and food security in China. Resour. Conserv. Recycl. 2021, 164, 105159. [Google Scholar] [CrossRef]
- Ji, J.L.; Wu, Y.; Xu, F.R.; Ju, X.R.; Wang, L.F. Physicochemical characteristics, composition and oxidative stability of Cucumis bisexualis seed oil. Food Sci. 2020, 41, 15–21. [Google Scholar]
- Hui, L.; Xu, W.; Chen, W.; Zhou, Z.; Li, J.; Ma, T.; Feng, S.; Xu, Y.; Zhao, T.; Yang, H. Characterization of the complete chloroplast genome of Cucun mismelo L. var. Agrestis Naud. Mitochondrial DNA Part B 2020, 5, 2744–2745. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Bursać Kovačević, D.; Zeković, Z.; Šojić, B.; Mrkonjić, Z.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and conventional valorizations of grape seeds from winery by-products as sustainable source of lipophilic antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Teslić, N.; Bojanić, N.; Čolović, D.; Fišteš, A.; Rakić, D.; Solarov, M.B.; Zeković, S.Z.; Pavlić, B. Conventional versus novel extraction techniques for wheat germ oil recovery: Multi-response optimization of supercritical fluid extraction. Sep. Sci. Technol. 2021, 56, 1546–1561. [Google Scholar] [CrossRef]
- Marić, B.; Pavlić, B.; Čolović, D.; Abramović, B.; Zeković, Z.; Bodroža-Solarov, M.; Teslić, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT 2020, 130, 109627. [Google Scholar] [CrossRef]
- Alrashidi, M.; Derawi, D.; Salimon, J.; Yusoff, M.F. An investigation of physicochemical properties of Nigella sativa L. Seed oil from Al-Qassim by different extraction methods. J. King Saud Univ. Sci. 2020, 32, 3337–3342. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, S.; Liu, H.; Li, B.-Z.; Che, L. Constituents and thermal properties of milk thistle seed oils extracted with three methods. LWT 2020, 126, 109282. [Google Scholar] [CrossRef]
- Wei, C.Q.; Liu, W.Y.; Xi, W.P.; Cao, D.; Zhang, H.J.; Ding, M.; Chen, L.; Xu, Y.Y.; Huang, K.X. Comparison of volatile compounds of hot-pressed, cold-pressed and solvent-extracted flaxseed oils analyzed by SPME-GC/MS combined with electronic nose: Major volatiles can be used as markers to distinguish differently processed oils. Eur. J. Lipid Sci. Technol. 2015, 117, 320–330. [Google Scholar] [CrossRef]
- Dierkes, G.; Bongartz, A.; Guth, H.; Hayen, H. Quality Evaluation of Olive Oil by Statistical Analysis of Multicomponent Stable Isotope Dilution Assay Data of Aroma Active Compounds. J. Agric. Food Chem. 2011, 60, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Zhao, P.; Zhang, T.; Wang, Y.; Guo, X.; Liu, R.; Jin, Q.Z.; Wang, X.G. Characteristic volatiles fingerprints and profiles determination in different grades of coconut oil by HS-GC-IMS and HS-SPME-GC-MS. Int. J. Food Sci. Technol. 2020, 55, 3670–3679. [Google Scholar] [CrossRef]
- Damiani, T.; Cavanna, D.; Serani, A.; Dall’Asta, C.; Suman, M. GC-IMS and FGC-Enose fingerprint as screening tools for revealing extra virgin olive oil blending with soft-refined olive oils: A feasibility study. Microchem. J. 2020, 159, 105374. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Capar, T.D.; Dedebas, T.; Yalcin, H.; Ekici, L. Extraction method affects seed oil yield, composition, and antioxidant properties of European cranberrybush (Viburnum opulus). Ind. Crop. Prod. 2021, 168, 113632. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of the compounds and characteristics of pepper seed oil by pressure-assisted, ultrasound-assisted and conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2019, 54, 78–86. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ghazani, S.M.; Corazza, M.L.; Marangoni, A.G.; Ribani, R.H. Assessment of subcritical propane, supercritical CO2 and Soxhlet extraction of oil from sapucaia (Lecythis pisonis) nuts. J. Supercrit. Fluids 2018, 133, 122–132. [Google Scholar] [CrossRef]
- Péres, V.F.; Saffi, J.; Melecchi, M.I.S.; Abad, F.C.; de Assis Jacques, R.; Martinez, M.M.; Oliveira, E.C.; Caramão, E.B. Comparison of soxhlet, ultrasound-assisted and pressurized liquid extraction of terpenes, fatty acids and Vitamin E from Piper gaudichaudianum Kunth. J. Chromatogr. A 2006, 1105, 115–118. [Google Scholar] [CrossRef]
- Satriana, S.; Supardan, M.D.; Arpi, N.; Mustapha, W.A.W. Development of Methods Used in the Extraction of Avocado Oil. Eur. J. Lipid Sci. Technol. 2018, 121, 1800210. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-Y.; Li, J.; Wang, M.; Zou, X.-G.; Peng, B.; Yin, Y.-L.; Deng, Z.-Y. A Novel Aqueous Extraction for Camellia Oil by Emulsified Oil: A Frozen/Thawed Method. Eur. J. Lipid Sci. Technol. 2019, 121, 1800431. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y.; Zong, Y.; Chu, Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochemistry 2021, 74, 105578. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S.; et al. A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crops Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Rutkowska, J.; Antoniewska, A.; Martinez-Pineda, M.; Nawirska-Olszańska, A.; Zbikowska, A.; Baranowski, D. Black Chokeberry Fruit Polyphenols: A Valuable Addition to Reduce Lipid Oxidation of Muffins Containing Xylitol. Antioxidants 2020, 9, 394. [Google Scholar] [CrossRef]
- Zhong, Z.C.; Kan, J.T.; Yuan, L. Effects of different extraction methods on the properties of Tibet Prunus mira nut oils. Chin. Oils Fats 2021, 4, 20–25. [Google Scholar]
- Shi, L.; Zheng, L.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Chemical Characterization, Oxidative Stability, and In Vitro Antioxidant Capacity of Sesame Oils Extracted by Supercritical and Subcritical Techniques and Conventional Methods: A Comparative Study Using Chemometrics. Eur. J. Lipid Sci. Technol. 2017, 120, 1700326. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Galleguillos-Fernández, R.; González-Barriga, V.; Valenzuela, R.; Speisky, H.; Fuentes, J.; Valenzuela, A. Fatty acid profile and bioactive compound extraction in purple viper’s bugloss seed oil extracted with green solvents. J. Am. Oil Chem. Soc. 2020, 97, 319–327. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. Ultrasonic acoustic-cavitation as a novel and emerging energy efficient technique for oil extraction from kapok seeds. Innov. Food Sci. Emerg. Technol. 2020, 62, 102347. [Google Scholar] [CrossRef]
- Ananth, D.A.; Deviram, G.; Mahalakshmi, V.; Sivasudha, T.; Tietel, Z. Phytochemical composition and antioxidant characteristics of traditional cold pressed seed oils in South India. Biocatal. Agric. Biotechnol. 2019, 17, 416–421. [Google Scholar] [CrossRef]
- Gu, L.-B.; Zhang, G.-J.; Du, L.; Du, J.; Qi, K.; Zhu, X.-L.; Zhang, X.-Y.; Jiang, Z.-H. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method. LWT 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.M.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboa, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Technol. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Monitoring storage shelf life of tomato using electronic nose technique. J. Food Eng. 2008, 85, 625–631. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Zhu, C.; Ma, X.; Rong, Y. Fatty acids and flavour components in the oil extracted from golden melon seeds. Eur. J. Lipid Sci. Technol. 2021, 123, 2000233. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.Q.; Liu, C.S.; Huang, F.H.; Huang, Y. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-nose and HS-SPME-GC×GC-TOF/MS. J. Food Biochem. 2019, 43, 12786. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Zhou, Y.; Chen, J. Effects of different preheat treatments on volatile compounds of camellia (Camellia oleifera Abel.) seed oil and formation mechanism of key aroma compounds. J. Food Biochem. 2021, 45, e13649. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Amador, R.M.; Fregapane, G.; Salvador, M.D. Influence of cultivar and technological conditions on the volatile profile of virgin pistachio oils. Food Chem. 2020, 311, 125957. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczynska-Sekowska, E.; Ścibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]





| Determination | Oils | |||
|---|---|---|---|---|
| PE | OSE | SE | AE | |
| Extraction yield (%) | 22.90 ± 0.24 bc | 24.64 ± 0.14 b | 34.47 ± 0.39 a | 18.57 ± 0.39 c |
| Acid value (mg KOH/g) | 0.676 ± 0.010 b | 0.224 ± 0.001 d | 0.448 ± 0.157 c | 2.349 ± 0.004 a |
| Peroxide value (m eq O2/kg) | 1.18 ± 0.05 b | 0.58 ± 0.10 c | 0.67 ± 0.09 c | 3.61 ± 0.35 a |
| Saponification value (mg KOH/g) | 191.91 ± 5.90 b | 223.52 ± 6.76 a | 215.44 ± 3.54 a | 227.42 ± 6.32 a |
| Iodine value (g I/100 g) | 104.42 ± 0.04 c | 114.71 ± 0.96 b | 126.48 ± 1.13 a | 111.82 ± 0.37 b |
| α-Tocopherol (mg/100 g) | 1.25 ± 0.03 d | 1.33 ± 0.21 c | 2.14 ± 0.12 a | 1.88 ± 0.06 b |
| β-Tocopherol (mg/100 g) | 1.25 ± 0.05 d | 1.55 ± 0.01 c | 2.71 ± 0.17 a | 2.21 ± 0.06 b |
| γ-Tocopherol (mg/100 g) | 11.26 ± 1.30 c | 14.79 ± 1.18 b | 20.63 ± 0.14 a | 7.56 ± 0.08 d |
| Property | Content (%) | |||
|---|---|---|---|---|
| PE | OSE | SE | AE | |
| Saturated fatty acid (SFAs) | ||||
| Palmitic acid (C16:0) | 7.00 ± 0.54 a | 2.73 ± 0.25 c | 5.01 ± 0.01 b | 2.62 ± 0.17 c |
| Stearic acid (C18:0) | 6.78 ± 0.22 a | 3.62 ± 0.14 b | 3.64 ± 0.47 b | 2.82 ± 0.11 c |
| Behenic acid (C22:0) | 6.14 ± 0.47 b | 2.83 ± 0.14 d | 10.16 ± 0.77 a | 3.51 ± 0.15 c |
| Lignoceric acid (C24:0) | 1.37 ± 0.02 c | 1.61 ± 0.24 b | 0.65 ± 0.04 d | 1.87 ± 0.07 a |
| Eicosanoic acid (C20:0) | 0.84 ± 0.00 c | 5.76 ± 0.44 a | 0.22 ± 0.0 d | 4.78 ± 0.41 b |
| Unsaturated fatty acid (UFAs) | ||||
| Monosaturated Fatty Acid (MUFAs) | ||||
| Oleic acid (C18:1) | 49.60 ± 0.45 b | 62.12 ± 0.14 a | 55.61 ± 0.52 b | 54.99 ± 1.14 b |
| Palmitoleic acid (C16:1) | 1.02 ± 0.23 c | 1.47 ± 0.04 b | 0.11 ± 0.00 d | 2.73 ± 0.11 a |
| Polysaturated fatty acids (PUFAs) | ||||
| Linoleic acid (C18:2) | 26.60 ± 0.32 a | 17.36 ± 0.78 c | 24.07 ± 0.88 a | 23.26 ± 1.03 b |
| Linolenic acid (C18:3) | 0.67 ± 0.08 c | 2.51 ± 0.10 b | 0.53 ± 0.03 c | 3.43 ± 0.17 a |
| SFAs | 22.12 ± 2.36 a | 16.55 ± 1.14 c | 19.68 ± 0.25 b | 15.59 ± 0.23 d |
| MUFAs | 50.62 ± 0.52 c | 63.59 ± 1.23 a | 55.72 ± 0.47 b | 57.71 ± 0.25 b |
| PUFAs | 27.27 ± 1.53 a | 19.86 ± 0.23 b | 24.60 ± 0.22 c | 26.70 ± 1.47 a |
| PUFA/SFA | 1.23 ± 0.04 b | 1.20 ± 0.03 b | 1.25 ± 0.47 b | 1.71 ± 0.23 a |
| Compound Name | CAS | RI | Molecular Formula | Content (%) | |||
|---|---|---|---|---|---|---|---|
| PE | OSE | SE | AE | ||||
| Hydrocarbons | |||||||
| Pentane | 109-66-0 | 518 | C5H12 | 1.03 ± 0.08 | 58.45 ± 1.56 | 26.00 ± 0.45 | |
| 3-Methyl-pentane | 96-14-0 | 554 | C6H14 | 13.11 ± 0.28 | 0.36 ± 0.08 | 0.37 ± 0.01 | |
| N-Hexane | 110-54-3 | 618 | C6H14 | 5.21 ± 0.12 | 62.34 ± 3.21 | ||
| Nethyl-cyclopentane | 96-37-7 | 661 | C6H12 | 39.64 ± 0.25 | 3.92 ± 0.01 | 0.27 ± 0.01 | |
| 8-Oxabicyclo octane | 286-45-3 | 850 | C7H12O | 1.44 ± 0.12 | 0.15 ± 0.01 | ||
| Hexacosane | 630-01-3 | 2606 | C26H54 | 0.30 ± 0.03 | |||
| Cyclopentane | 287-92-3 | 600 | C5H10 | 1.62 ± 0.05 | 11.16 ± 0.04 | ||
| Cyclohexane | 110-82-7 | 618 | C6H14 | 2.66 ± 0.24 | |||
| 2-Nethyl-hexane, | 591-76-4 | 653 | C7H16 | 1.78 ± 0.04 | |||
| 2,3-Dimethyl-pentane, | 565-59-3 | 589 | C7H16 | 0.74 ± 0.07 | |||
| 1,1-Dimethyl-cyclopentane | 1638-26-2 | 734 | C7H14 | 0.14 ± 0.00 | |||
| 3-Dethyl-hexane, | 589-34-4 | 653 | C7H16 | 1.89 ± 0.45 | |||
| 1,3-Dimethyl-cyclopentane | 2532-58-3 | 722 | C7H14 | 0.86 ± 0.14 | |||
| Heptane | 142-82-5 | 717 | C7H16 | 1.38 ± 0.01 | |||
| Cyclohexane, methyl- | 108-87-2 | 781 | C7H14 | 0.54 ± 0.07 | |||
| Undecane | 1120-21-4 | 1115 | C11H24 | 1.15 ± 0.04 | 0.98 ± 0.11 | ||
| Dodecane | 112-40-3 | 1214 | C12H26 | 1.22 ± 0.08 | |||
| Nonane, 3-methyl-5-propyl- | 629-59-4 | 1413 | C13H28 | 0.52 ± 0.01 | |||
| Octane | 111-65-9 | 816 | C8H18 | 1.48 ± 0.47 | 0.52 ± 0.01 | ||
| Heneicosane | 629-94-7 | 2109 | C21H44 | 0.87 ± 0.11 | |||
| 5-(2-Methylpropyl)-Nonane | 62185-53-9 | 1185 | C13H28 | 0.34 ± 0.01 | |||
| Sum | 5.41 ± 0.45 | 55.79 ± 1.07 | 91.81 ± 0.97 | 89.43 ± 1.45 | |||
| Esters | |||||||
| (S)-l-Alanine ethylamide | 17344-99-9 | 864 | C5H11NO2 | 0.09 ± 0.00 | 0.87 ± 0.01 | ||
| Dibutyl phthalate | 84-74-2 | 2037 | C16H22O4 | 2.41 ± 0.12 | 0.46 ± 0.0.2 | ||
| Formic acid, hexyl ester | 629-33-4 | 981 | C7H14O2 | 0.41 ± 0.00 | 0.05 ± 0.00 | ||
| Docosanoic acid nonyl ester | 42233-05-6 | 3270 | C31H62O2 | 0.50 ± 0.01 | |||
| Decanoic acid, decyl ester | 1654-86-0 | 2177 | C20H40O2 | 2.68 ± 0.04 | |||
| 2-Ethylhexyl acrylate | 103-11-7 | 1208 | C11H20O2 | 5.68 ± 0.35 | |||
| Sum | 5.59 ± 0.25 | 6.09 ± 0.35 | 0.14 ± 0.01 | 1.33 ± 0.21 | |||
| Aldehydes | |||||||
| 2-Methyl-butanal | 96-17-3 | 643 | C5H10O | 1.31 ± 0.11 | 0.08 ± 0.01 | ||
| Hexanal | 66-25-1 | 806 | C6H12O | 23.07 ± 0.14 | 3.48 ± 0.24 | ||
| Benzaldehyde | 100-52-7 | 982 | C7H6O | 2.02 ± 0.01 | 0.34 ± 0.01 | ||
| 3-Methyl-butanal | 590-86-3 | 643 | C5H10O | 4.87 ± 0.48 | |||
| Pentanal | 110-62-3 | 707 | C5H10O | 2.99 ± 0.24 | |||
| Decanal | 112-31-2 | 1204 | C10H20O | 2.17 ± 0.25 | |||
| Sum | 35.12 ± 0.57 | 0.00 | 1.31 ± 0.11 | 3.90 ± 0.33 | |||
| Alkenes | |||||||
| 1-Pentene | 109-67-1 | 508 | C5H10 | 0.10 ± 0.01 | 0.42 ± 0.14 | ||
| Styrene | 100-42-5 | 883 | C8H8 | 2.34 ± 0.23 | 33.33 ± 0.54 | 1.00 ± 0.07 | |
| (Z)-3-Methyl-2-undecene | 19780-34-8 | 1199 | C12H24 | 0.65 ± 0.01 | |||
| Trichloroethylene | 1979/1/6 | 734 | C2HCl3 | 2.12 ± 0.24 | |||
| Sun | 2.34 ± 23 | 33.43 ± 0.78 | 2.77 ± 0.36 | 1.42 ± 0.18 | |||
| Ketones | |||||||
| 2-Methyl-4-heptanone | 626-33-5 | 888 | C8H16O | 1.54 ± 0.05 | |||
| Acetyl valeryl | 96--04-8 | 989 | C7H12O2 | 0.06 ± 0.00 | |||
| (R)-(+)-3-Methylcyclopentanone | 6672-30-6 | 832 | C6H10O | 0.33 ± 0.04 | |||
| 5-Methyl-2-hexanone | 110-12-3 | 789 | C7H14O | 4.43 ± 0.11 | |||
| 3-Methyl-2-butanone | 563-80-4 | 590 | C5H10O | 1.88 ± 0.01 | |||
| 2-Butanone | 78-93-3 | 555 | C4H8O | 0.89 ± 0.04 | |||
| Acetoin | 513-86-0 | 717 | C4H8O2 | 0.74 ± 0.02 | |||
| 2-Methyl-3-heptanone | 13019-20-0 | 888 | C8H16O | 14.51 ± 0.07 | 2.19 ± 0.88 | ||
| Sum | 22.45 ± 0.19 | 2.19 ± 0.88 | 0.39 ± 0.04 | 1.54 ± 0.05 | |||
| Alcohols | |||||||
| Isopropyl alcohol | 67-63-0 | 482 | C3H8O | 3.50 ± 0.44 | |||
| (R)-2-Butanol, | 14898-79-4 | 581 | C4H10O | 2.68 ± 0.04 | |||
| 1-Butanol | 71-36-3 | 662 | C4H10O | 5.46 ± 0.48 | |||
| [R-(R*,R*)]-2,3-Butanediol | 24347-58-8 | 743 | C4H10O2 | 0.57 ± 0.04 | |||
| 1-Heptacosanol | 2004-39-9 | 2948 | C27H56O | 2.91 ± 0.57 | |||
| Eucalyptol | 470-82-6 | 1059 | C10H18O | 1.68 ± 0.22 | 0.29 ± 0.01 | 0.28 ± 0.00 | |
| Sum | 16.8 ± 1.01 | 0.29 ± 0.01 | 0.00 | 0.28 ± 0.00 | |||
| Acids | |||||||
| Butanoic acid | 107-92-6 | 775 | C4H8O2 | 0.82 ± 0.06 | |||
| Hexanoic acid | 142-62-1 | 974 | C6H12O2 | 2.59 ± 0.45 | |||
| Tetradecanoic acid | 544-63-8 | 1769 | C14H28O2 | 0.53 ± 0.06 | |||
| Sum | 3.94 ± 0.57 | 0 | 0 | 0 | |||
| Other | |||||||
| 2-Pentyl-furan | 3777-69-3 | 1040 | C9H14O | 7.24 + 0.48 | 0.61 ± 0.01 | ||
| Tetrahydrofuran | 109-99-9 | 589 | C4H8O | 1.11 ± 0.04 | |||
| 1,3-Dimethyl-benzene | 108-38-3 | 907 | C8H10 | 1.85 ± 0.22 | 0.19 ± 0.01 | ||
| O-Xylene | 95-47-6 | 907 | C8H10 | 0.97 ± 0.12 | 1.30 ± 0.14 | ||
| Toluene | 108-88-3 | 794 | C7H8 | 0.99 ± 0.04 | |||
| Ethylbenzene | 100-41-4 | 893 | C8H10 | 0.19 ± 0.04 | |||
| 1-Ethyl-2-methyl-benzene | 611-14-3 | 1006 | C9H12 | 0.73 ± 0.11 | 0.55 + 0.09 | ||
| 1-Ethyl-4-methyl-benzene | 622-96-8 | 1006 | C9H12 | 0.19 ± 0.01 | |||
| 1,2,3-Trimethyl-benzene | 526-73-8 | 1020 | C9H12 | 0.32 ± 0.1 | |||
| Sum | 8.35 ± 0.55 | 2.21 ± 0.21 | 3.58 ± 0.01 | 2.10 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yuan, Y.; Zhu, X.; Xu, R.; Shen, H.; Zhang, Q.; Ge, X. The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil. Foods 2022, 11, 721. https://doi.org/10.3390/foods11050721
Zhang H, Yuan Y, Zhu X, Xu R, Shen H, Zhang Q, Ge X. The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil. Foods. 2022; 11(5):721. https://doi.org/10.3390/foods11050721
Chicago/Turabian StyleZhang, Huijun, Yushu Yuan, Xiuxiu Zhu, Runzhe Xu, Huishan Shen, Qian Zhang, and Xiangzhen Ge. 2022. "The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil" Foods 11, no. 5: 721. https://doi.org/10.3390/foods11050721