Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals
Abstract
:1. Introduction
2. Materials and Methods
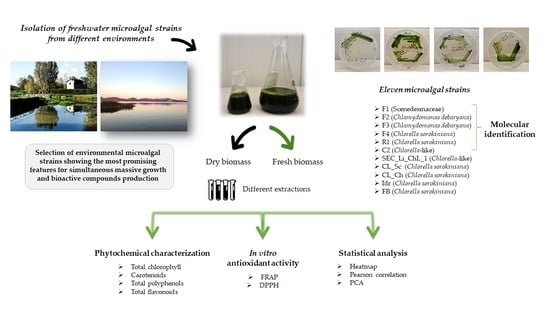
2.1. Microalgal Strain Isolation
2.1.1. Sample Collection
2.1.2. Isolation of Microalgal Strains from Collected Samples
2.1.3. Cultivation of Microalgae
2.2. Molecular Identification of the Microalgal Strains
2.3. Microalgal Sample Extraction
2.4. Microalgal Phytochemical Composition
2.4.1. Photosynthetic Pigment Content and Analysis
2.4.2. Total Polyphenol and Flavonoid Content
2.5. In Vitro Antioxidant Activities
2.5.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.2. Radical Scavenging Activity
2.6. Statistical Analysis
3. Results
3.1. Molecular Identification of the Microalgal Strains
3.2. Quantification of Photosynthetic Pigments
3.3. Total Polyphenol and Flavonoid Content
3.4. In Vitro Antioxidant Activities
3.5. Statistical Analysis Overview
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, M.P.; Hoeltz, M.; Gressler, P.D.; Benitez, L.B.; Schneider, R.C.S. Potential of Microalgal Bioproducts: General Perspectives and Main Challenges. Waste Biomass Valoriz. 2019, 10, 2139–2156. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Colla, E.; Menegotto, A.L.L.; Kalschne, D.L.; da Silva-Buzanello, R.A.; Canan, C.; Drunkler, D.A. Chapter 32-Microalgae: A new and promising source of food. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Academic Press: New York, NY, USA, 2020; pp. 507–518. [Google Scholar]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayan, A.; Arumugam, M.; Pandey, A. Chapter 4-Nutraceuticals From Algae and Cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Sousa, I.; Gouveia, L.; Batista, A.; Raymundo, A.; Bandarra, N. Chapter 2. Microalgae in novel food products. Algae Nutr. Pollut. Control. Energy Sources 2008, 75–112. [Google Scholar]
- Gabriele, M.; Pucci, L. Diet Bioactive Compounds: Implications for Oxidative Stress and Inflammation in the Vascular System. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 264–275. [Google Scholar] [CrossRef]
- Zhao, Z.; Rasool, M.A.; Chen, C.; Ma, S.; Wang, L.; Huang, G. Identification and screening of multiple tropical microalgal strains for antioxidant activity in vitro. Food Biosci. 2020, 36, 100649. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic Profile and Antioxidant Activity of Crude Extracts from Microalgae and Cyanobacteria Strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, M. Microalgae Nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Yatipanthalawa, B.; Martin, G. Chapter 3-Conventional and novel approaches to extract food ingredients and nutraceuticals from microalgae. In Cultured Microalgae for the Food Industry; Lafarga, T., Acién, G., Eds.; Academic Press: New York, NY, USA, 2021; pp. 73–96. [Google Scholar]
- Castro-Muñoz, R.; García-Depraect, O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, C.F.; Henry, B.S. Plant pigments as natural food colours. Endeavour 1986, 10, 31–36. [Google Scholar] [CrossRef]
- Vesenick, D.; Paula, N.; Niwa, A.; Mantovani, M. Evaluation of the Effects of Chlorophyllin on Apoptosis Induction, Inhibition of Cellular Proliferation and mRNA Expression of CASP8, CASP9, APC and $-catenin. Curr. Res. J. Biol. Sci. 2012, 4, 315–322. [Google Scholar]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.d.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Tarik, A.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Kesavan, P.; Banerjee, A.; Banerjee, A.; Murugesan, R.; Marotta, F.; Pathak, S. Chapter 17. An Overview of Dietary Polyphenols and Their Therapeutic Effects. Polyphen. Mech. Action Hum. Health Dis. 2018, 221–235. [Google Scholar] [CrossRef]
- Freile-Pelegrin, Y.; Robledo, D. Chapter 6. Bioactive phenolic compounds from algae. Bioact. Compd. Mar. Foods Plant. Anim. Sources 2014, 113–129. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- Islam, M.N.; Alsenani, F.; Schenk, P. Chapter 1. Microalgae as a Sustainable Source of Nutraceuticals. Microb. Funct. Foods Nutraceuticals 2017, 1–18. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Chiellini, C.; Guglielminetti, L.; Sarrocco, S.; Ciurli, A. Isolation of Four Microalgal Strains From the Lake Massaciuccoli: Screening of Common Pollutants Tolerance Pattern and Perspectives for Their Use in Biotechnological Applications. Front. Plant Sci. 2020, 11, 607651. [Google Scholar] [CrossRef] [PubMed]
- Ciurli, A.; Modeo, L.; Pardossi, A.; Chiellini, C. Multidisciplinary integrated characterization of a native Chlorella-like microalgal strain isolated from a municipal landfill leachate. Algal Res. 2021, 54, 102202. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [Green Version]
- Saba, F.; Papizadeh, M.; Khansha, J.; Sedghi, M.; Rasooli, M.; Amoozegar, M.; Soudi, M.; Shahzadeh Fazeli, S.A. A Rapid and Reproducible Genomic DNA Extraction Protocol for Sequence-Based Identification of Archaea, Bacteria, Cyanobacteria, Diatoms, Fungi, and Green Algae. J. Med. Bacteriol. 2016, 5, 22–28. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: New York, NY, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical Composition and Antioxidant Activity of Tuscan Bee Pollen of Different Botanic Origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar] [CrossRef]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The effect of sourdough fermentation on Triticum dicoccum from Garfagnana: 1H NMR characterization and analysis of the antioxidant activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.r-project.org/ (accessed on 20 January 2022).
- Kolde, R. Pheatmap: Pretty Heatmaps. 2019. R Package Version 1.0.12 [Software]. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 20 January 2022).
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Alam, M.A.; Xu, J.-L.; Wang, Z. Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020. [Google Scholar]
- Bao, B.; Thomas-Hall, S.R.; Schenk, P.M. Fast-Tracking Isolation, Identification and Characterization of New Microalgae for Nutraceutical and Feed Applications. Phycology 2022, 2, 86–107. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process. Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process. Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Chia, M.; Lombardi, A.; Melão, M. Growth and biochemical composition of Chlorella vulgaris in different growth media. An. Da Acad. Bras. De Cienc. 2013, 85, 1427–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards green extraction methods from microalgae learning from the classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rinawati, M.; Sari, L.; Pursetyo, K. Chlorophyll and carotenoids analysis spectrophotometer using method on microalgae. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012056. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Santhakumaran, P.; Ayyappan, S.M.; Ray, J.G. Nutraceutical applications of twenty-five species of rapid-growing green-microalgae as indicated by their antibacterial, antioxidant and mineral content. Algal Res. 2020, 47, 101878. [Google Scholar] [CrossRef]
- Tiong, I.K.R.; Nagappan, T.; Abdul Wahid, M.E.; Tengku Muhammad, T.S.; Tatsuki, T.; Satyantini, W.H.; Mahasri, G.; Sorgeloos, P.; Sung, Y.Y. Antioxidant capacity of five microalgae species and their effect on heat shock protein 70 expression in the brine shrimp Artemia. Aquac. Rep. 2020, 18, 100433. [Google Scholar] [CrossRef]
- Mfotie Njoya, E. Medicinal Plants, Antioxidant Potential, and Cancer; Academic Press: New York, NY, USA, 2021; pp. 349–357. [Google Scholar]
- Hajimahmoodi, M.; Faramarzi, M.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Goh, S.-H.; Yusoff, F.M.; Loh, S.P. A Comparison of the Antioxidant Properties and Total Phenolic Content in a Diatom, Chaetoceros sp. and a Green Microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, 123. [Google Scholar] [CrossRef] [Green Version]



| Strain | Isolation Source | Sequenced Gene | Accession Number | Length (bp) | Best Blast Hit (Identity %) | Taxonomic Affiliation |
|---|---|---|---|---|---|---|
| F1 | “Le Morette”, Fucecchio Marshland | 18S rDNA | OM311002 | 2919 | Desmodesmus brasiliensis AB917106 (94.53) | Scenedesmaceae |
| Final portion of 18S, complete ITS1-5.8S-ITS2, initial portion of 28S rDNA | OM310999 | 1153 | Desmodesmus subspicatus MK975481 (96.63) | |||
| F2 | “Le Morette”, Fucecchio Marshland | 18S rDNA | OM311003 | 1703 | Chlamydomonas debaryana MF678003 (99.82) | Chlamydomonas debaryana |
| F3 | “Le Morette”, Fucecchio Marshland | 18S rDNA | OM311004 | 1716 | Chlamydomonas debaryana MF678003 (99.94) | Chlamydomonas debaryana |
| F4 | “Le Morette”, Fucecchio Marshland | 18S rDNA | OM311005 | 1758 | Chlorella sorokiniana MF101221 (100) | Chlorella sorokiniana |
| Final portion of 18S, complete ITS1-5.8S-ITS2, initial portion of 28S rDNA | OM311000 | 738 | Chlorella sorokiniana KM514859 (99.59) | |||
| R1 | Private terrace in Pisa, water sample | 18S rDNA | OM311006 | 1758 | Chlorella sorokiniana MF101221 (99.89) | Chlorella sorokiniana |
| C2 | Heating system of a private house | 18S rDNA | OM311001 | 1704 | Chlorella sp. KP262476 (99.30) | Chlorella-like microalgal strain |
| Final portion of 18S, complete ITS1-5.8S-ITS2, initial portion of 28S rDNA | OM310998 | 1279 | Chlorella vulgaris KT778121 (99.70) |
| p-Value | Carotenoids | Polyphenols | Flavonoids | DPPH | FRAP |
|---|---|---|---|---|---|
| R2 | |||||
| Carotenoids | 0.021 | 2.51 × 10−4 | 4.12 × 10−5 | 6.46 × 10−5 | |
| Polyphenols | 0.400 | 2.32 × 10−4 | 2.7 × 10−4 | 5.45 × 10−4 | |
| Flavonoids | 0.596 | 0.539 | 0.004 | 4.31 × 10−10 | |
| DPPH | 0.651 | 0.523 | 0.430 | 4.27 × 10−6 | |
| FRAP | 0.638 | 0.506 | 0.798 | 0.638 |
| p-Value | Carotenoids | Polyphenols | Flavonoids | DPPH | FRAP |
|---|---|---|---|---|---|
| R2 | |||||
| Carotenoids | 0.738 | 0.939 | 0.624 | 0.41 | |
| Polyphenols | −0.061 | 0.065 | 0.393 | 0.041 | |
| Flavonoids | −0.014 | 0.3259 | 0.002 | 0.001 | |
| DPPH | 0.089 | 0.147 | 0.505 | 5.013 × 10−11 | |
| FRAP | 0.1489 | 0.337 | 0.549 | 0.827 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiellini, C.; Serra, V.; Gammuto, L.; Ciurli, A.; Longo, V.; Gabriele, M. Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals. Foods 2022, 11, 654. https://doi.org/10.3390/foods11050654
Chiellini C, Serra V, Gammuto L, Ciurli A, Longo V, Gabriele M. Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals. Foods. 2022; 11(5):654. https://doi.org/10.3390/foods11050654
Chicago/Turabian StyleChiellini, Carolina, Valentina Serra, Leandro Gammuto, Adriana Ciurli, Vincenzo Longo, and Morena Gabriele. 2022. "Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals" Foods 11, no. 5: 654. https://doi.org/10.3390/foods11050654