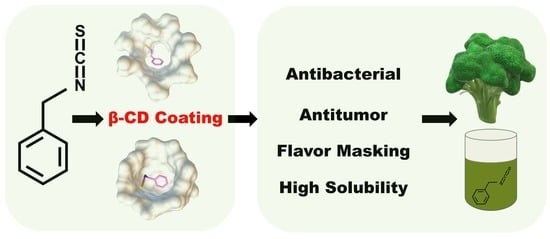
Encapsulation of Benzyl Isothiocyanate with β-Cyclodextrin Using Ultrasonication: Preparation, Characterization, and Antibacterial Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
Materials
2.2. Molecular Modeling
2.3. Solubility of BITC in β-CD Derivatives
2.4. Preparation of BITC Inclusion Complexes
2.5. Determination of Inclusion Rate
2.6. Determination of Inclusion Stability
2.7. Particle Size Distribution
2.8. Infrared Spectroscopy (IR)
2.9. Differential Scanning Calorimetry (DSC)
2.10. 1H Nuclear Magnetic Resonance (1H-NMR)
2.11. Antibacterial Activities
2.12. Antitumor Activities
2.13. Antimicrobial Activity during Broccoli Juice Pasteurization
2.14. Statistical Analysis
3. Results and Discussion
3.1. Molecular Modeling
3.2. Solubilization of BITC
3.3. Embedding Rate and Preservation Rate of BITC
3.4. Particle Size Analysis
3.5. FT-IR Spectral Studies
3.6. Thermodynamics Analysis
3.7. 1H-NMR Analysis
3.8. Anti-Bacterial and Anti-Tumor Activity
3.9. Antimicrobial Stability of BITC-β-CDs in Broccoli Juice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, A.; Biehler, D.; Nobis, T.; Richter, H.; Engels, I.; Biehler, K.; Frank, U. Broad spectrum antibacterial activity of a mixture of isothiocyanates from nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix). Drug Res. 2013, 63, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An overview of their antimicrobial activity against human infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [Green Version]
- Uppal, S.; Sharma, P.; Kumar, R.; Kaur, K.; Bhatia, A.; Mehta, S.K. Effect of benzyl isothiocyanate encapsulated biocompatible nanoemulsion prepared via ultrasonication on microbial strains and breast cancer cell line MDA MB 231. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124732. [Google Scholar] [CrossRef]
- do Nascimento, E.G.; de Azevedo, E.P.; Alves-Silva, M.F.; Aragão, C.F.S.; Fernandes-Pedrosa, M.F.; da Silva-Junior, A.A. Supramolecular aggregates of cyclodextrins with co-solvent modulate drug dispersion and release behavior of poorly soluble corticosteroid from chitosan membranes. Carbohydr. Polym. 2020, 248, 116724. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Arima, H.; Motoyama, K.; Irie, T. Recent Findings on Safety Profiles of Cyclodextrins, Cyclodextrin Conjugates, and Polypseudorotaxanes; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 91–122. [Google Scholar] [CrossRef]
- Loftsson, T. Excipient pharmacokinetics and profiling. Int. J. Pharm. 2015, 480, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Du, J.; Zhang, J.; Du, L.; Fang, H.; Li, M. How to improve docking accuracy of AutoDock4. 2: A case study using different electrostatic potentials. J. Chem. Inf. Model. 2013, 53, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, J.; IKoay, A.; Di Paolo, A.; Bieri, M.; Petrie, E.J.; Gorman, M.A.; Doughty, L.; Parker, M.W.; Stapleton, D.I.; Griffin, M.D. Determinants of oligosaccharide specificity of the carbohydrate-binding modules of AMP-activated protein kinase. Biochem. J. 2015, 468, 245–257. [Google Scholar] [CrossRef]
- Schwarz, D.H.; Engelke, A.; Wenz, G. Solubilizing steroidal drugs by β-cyclodextrin derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.; Zhou, Y.; Bei, W.; Li, Y.; Yuan, Q.; Liang, H. Characterization of glabridin/hydroxypropyl-β-cyclodextrin inclusion complex with robust solubility and enhanced bioactivity. Carbohydr. Polym. 2017, 159, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Tang, B.; Wang, Q.; Xu, K.; Xiong, X.; Li, H. Effect of hydroxypropyl-β-cyclodextrin on the bounding of salazosulfapyridine to human serum albumin. Int. J. Biol. Macromol. 2016, 92, 105–115. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Inclusion complexation of catechin by β-cyclodextrins: Characterization and storage stability. LWT 2017, 86, 555–565. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Li, S.; Liang, N.; Yan, P.; Kawashima, Y.; Sun, S. Inclusion complex based on N-acetyl-L-cysteine and arginine modified hydroxypropyl-β-cyclodextrin for oral insulin delivery. Carbohydr. Polym. 2021, 252, 117202. [Google Scholar] [CrossRef]
- Li, H.; Ming, X.; Xu, D.; Mo, H.; Liu, Z.; Hu, L.; Zhou, X. Transcriptome Analysis and Weighted Gene Co-expression Network Reveal Multitarget-Directed Antibacterial Mechanisms of Benzyl Isothiocyanate against Staphylococcus aureus. J. Agric. Food Chem. 2021, 69, 11733–11741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Z.; Mo, H.; Zhao, Y.; Zhou, X. Thymol Mediates Bactericidal Activity against Staphylococcus aureus by Targeting an Aldo-Keto Reductase and Consequent Depletion of NADPH. J. Agric. Food Chem. 2019, 67, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Ww, A.; Wei, X.J.C.; Biointerfaces, S.B. Evaluation of anticancer activity of honokiol by complexation with hydroxypropyl-β-cyclodextrin. Colloids Surf. B Biointerf. 2020, 196, 111298. [Google Scholar] [CrossRef]
- Ren, L.; Wang, J.; Chen, G.J.D.D. Preparation, optimization of the inclusion complex of glaucocalyxin A with sulfobutylether-β-cyclodextrin and antitumor study. Drug Deliv. 2019, 26, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.F.; Chiang, N.N.; Lu, Y.H.; Huang, Y.S.; Yang, J.S.; Tsai, S.C.; Lu, C.C.; Chen, F.A. Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. BioMedicine 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S.K. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Hadian, Z.; Maleki, M.; Abdi, K.; Atyabi, F.; Mohammadi, A.; Khaksar, R. Preparation and Characterization of Nanoparticle beta-Cyclodextrin:Geraniol Inclusion Complexes. Iran. J. Pharm. Res. 2018, 17, 39–51. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kahlon, N.K.; Singh, R.; Mehta, S.K. Encompassment of Benzyl Isothiocyanate in cyclodextrin using ultrasonication methodology to enhance its stability for biological applications. Ultrason. Sonochem. 2017, 39, 25–33. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Ao, X.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Characterization of the Inclusion Complexes of Isothiocyanates with gamma-Cyclodextrin for Improvement of Antibacterial Activities against Staphylococcus aureus. Foods 2021, 11, 60. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Siva, S.; Lin, L. Ultrasound processed cuminaldehyde/2-hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization and antibacterial activity. Ultrason. Sonochem. 2019, 56, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Liu, J.-C.; Yang, W.-B.; Chen, D.-L.; Jiao, Z.-G. Experimental and molecular docking investigations on the inclusion mechanism of the complex of phloridzin and hydroxypropyl-β-cyclodextrin. Food Chem. 2017, 215, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Choi, J.U.; Panthi, V.K.; Byun, Y.; Park, J.W. Enhanced oral absorption of pemetrexed by ion-pairing complex formation with deoxycholic acid derivative and multiple nanoemulsion formulations: Preparation, characterization, and in vivo oral bioavailability and anticancer effect. Int. J. Nanomed. 2018, 13, 3329–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and evaluation of cyclodextrin complexes for improved anticancer activity of repurposed drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef]
- Burcul, F.; Generalic Mekinic, I.; Radan, M.; Rollin, P.; Blazevic, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yanaka, A.; Sato, J.; Ohmori, S. Sulforaphane protects small intestinal mucosa from aspirin/NSAID-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr. Pharm. Des. 2013, 19, 157–162. [Google Scholar] [CrossRef]
- Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. A Bioactive Chitosan-Based Film Enriched with Benzyl Isothiocyanate/alpha-Cyclodextrin Inclusion Complex and Its Application for Beef Preservation. Foods 2022, 11, 2687. [Google Scholar] [CrossRef]
- Kothe, C.I.; Pessoa, J.P.; Malheiros, P.S.; Tondo, E.C. Assessing the growth of Staphylococcus aureus and Escherichia coli on fruits and vegetables. J. Infect. Dev. Ctries. 2019, 13, 480–486. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ming, X.; Wang, Z.; Li, J.; Liang, Y.; Xu, D.; Liu, Z.; Hu, L.; Mo, H. Encapsulation of Benzyl Isothiocyanate with β-Cyclodextrin Using Ultrasonication: Preparation, Characterization, and Antibacterial Assay. Foods 2022, 11, 3724. https://doi.org/10.3390/foods11223724
Li H, Ming X, Wang Z, Li J, Liang Y, Xu D, Liu Z, Hu L, Mo H. Encapsulation of Benzyl Isothiocyanate with β-Cyclodextrin Using Ultrasonication: Preparation, Characterization, and Antibacterial Assay. Foods. 2022; 11(22):3724. https://doi.org/10.3390/foods11223724
Chicago/Turabian StyleLi, Hongbo, Xujia Ming, Zhen Wang, Jiaqi Li, Yunxia Liang, Dan Xu, Zhenbin Liu, Liangbin Hu, and Haizhen Mo. 2022. "Encapsulation of Benzyl Isothiocyanate with β-Cyclodextrin Using Ultrasonication: Preparation, Characterization, and Antibacterial Assay" Foods 11, no. 22: 3724. https://doi.org/10.3390/foods11223724