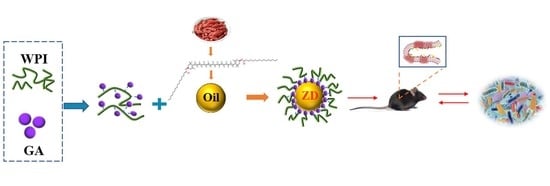
Zeaxanthin Dipalmitate-Enriched Emulsion Stabilized with Whey Protein Isolate-Gum Arabic Maillard Conjugate Improves Gut Microbiota and Inflammation of Colitis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZD Purification and Emulsions Preparation
2.2. Characterization of Emulsions
2.3. Mice Treatments
2.4. Determinations of Serum Biochemical Parameters
2.5. Histological Analysis
2.6. Immunofluorescence Staining
2.7. Determination of Short-Chain Fatty Acids (SCFAs)
2.8. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR) Analysis
2.9. 16S rDNA Gene High-Throughput Sequencing
2.10. Statistical Analysis
3. Results
3.1. WPI-GA and WPI-GA-ZD Relieved the Symptoms of DSS-Induced Colitis in Mice
3.2. WPI-GA and WPI-GA-ZD Decreased the Levels of Proinflammatory Cytokines and Endotoxins
3.3. WPI-GA and WPI-GA-ZD Reduced Histological Damage of Colon
3.4. WPI-GA and WPI-GA-ZD Improved the Levels of Tight Junction Proteins
3.5. WPI-GA-ZD Increased the SCFAs Contents
3.6. WPI-GA and WPI-GA-ZD Regulated the mRNA Expression of Recognition Receptors and Cytokines
3.7. WPI-GA and WPI-GA-ZD Regulated the Gut Microbiota Structure
3.8. Function Prediction of Gut Microbiota Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugihara, K.; Kamada, N. Diet–Microbiota Interactions in Inflammatory Bowel Disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.; Martinho-Grueber, M.; Studerus, D.; Vavricka, S.R.; Tilg, H.; Biedermann, L. Nutrition in Inflammatory Bowel Disease. Digestion 2020, 101, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The Role of Diet in the Aetiopathogenesis of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-Related Factors Explaining Interindividual Variability of Carotenoid Bioavailability and Tissue Concentrations in Humans. Mol. Nutr. Food Res. 2017, 61, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Sakai, S.; Nishida, A.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; Andoh, A. Astaxanthin, a Xanthophyll Carotenoid, Prevents Development of Dextran Sulphate Sodium-Induced Murine Colitis. J. Clin. Biochem. Nutr. 2019, 64, 66–72. [Google Scholar] [CrossRef] [Green Version]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin Exerts Protective Effects on Acetic Acid-Induced Colitis in Rats via Modulation of pro-Inflammatory Cytokines and Oxidative Stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Kan, X.; Yan, Y.; Ran, L.; Lu, L.; Mi, J.; Zhang, Z.; Zeng, X.; Cao, Y. Ultrasonic-Assisted Extraction and High-Speed Counter-Current Chromatography Purification of Zeaxanthin Dipalmitate from the Fruits of Lycium barbarum L. Food Chem. 2020, 310, 125854. [Google Scholar] [CrossRef]
- Kan, X.; Chen, G.; Zhou, W.; Zeng, X. Application of Protein-Polysaccharide Maillard Conjugates as Emulsifiers: Source, Preparation and Functional Properties. Food Res. Int. 2021, 150, 110740. [Google Scholar] [CrossRef]
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased Consumption of Emulsifiers as an Explanation for the Rising Incidence of Crohn’s Disease. J. Crohn’s Colitis 2013, 7, 338–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooshkam, M.; Varidi, M. Maillard Conjugate-Based Delivery Systems for the Encapsulation, Protection, and Controlled Release of Nutraceuticals and Food Bioactive Ingredients: A Review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Kan, X.; Hu, Y.; Huang, Y.; Fan, X.; Chen, G.; Ye, H.; Zeng, X. Characterization of Whey Protein Isolate-Gum Arabic Maillard Conjugate and Evaluation of the Effects of Conjugate-Stabilized Emulsion on Microbiota of Human Fecal Cultures. Food Hydrocoll. 2023, 134, 108060. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yajima, T.; Kubo, C.; Yoshikai, Y. Role of Interleukin 15 in Colitis Induced by Dextran Sulphate Sodium in Mice. Gut 2006, 55, 334–341. [Google Scholar] [CrossRef]
- Stillie, R.; Stadnyk, A.W. Role of TNF Receptors, TNFR1 and TNFR2, in Dextran Sodium Sulfate-Induced Colitis. Inflamm. Bowel Dis. 2009, 15, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, G.; Wan, P.; Hu, B.; Chen, L.; Ou, S.; Zeng, X.; Ye, H. Digestion under Saliva, Simulated Gastric and Small Intestinal Conditions and Fermentation in Vitro of Polysaccharides from the Flowers of Camellia sinensis Induced by Human Gut Microbiota. Food Funct. 2017, 8, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary Emulsifiers Directly Alter Human Microbiota Composition and Gene Expression Ex Vivo Potentiating Intestinal Inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-biroulet, L.; Colombel, J. Ulcerative Colitis. Lancet 2016, 6736, 1–15. [Google Scholar] [CrossRef]
- Dai, Z.; Feng, S.; Liu, A.; Wang, H.; Zeng, X.; Yang, C.S. Anti-Inflammatory Effects of Newly Synthesized α-Galacto-Oligosaccharides on Dextran Sulfate Sodium-Induced Colitis in C57BL/6J Mice. Food Res. Int. 2018, 109, 350–357. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Ameraoui, H.; Muccioli, G.G. Bioactive Lipids in Inflammatory Bowel Diseases—From Pathophysiological Alterations to Therapeutic Opportunities. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 158854. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xiong, Q.; Kong, J.; Tian, C.; Miao, L.; Zhang, X.; Du, H. Intraperitoneal Supplementation of Iron Alleviates Dextran Sodium Sulfate-Induced Colitis by Enhancing Intestinal Barrier Function. Biomed. Pharmacother. 2021, 144, 112253. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; Mullaney, J.A.; Kleerebezem, M.; Smidt, H.; Rosendale, D.I. Modulation of the Microbial Fermentation in the Gut by Fermentable Carbohydrates. Bioact. Carbohydrates Diet. Fibre 2013, 2, 133–142. [Google Scholar] [CrossRef]
- Engels, C.; Ruscheweyh, H.-J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium Hallii Also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Glauben, R.; Batra, A.; Fedke, I.; Zeitz, M.; Lehr, H.A.; Leoni, F.; Mascagni, P.; Fantuzzi, G.; Dinarello, C.A.; Siegmund, B. Histone Hyperacetylation Is Associated with Amelioration of Experimental Colitis in Mice. J. Immunol. 2006, 176, 5015–5022. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Environmental Risk Factors for Inflammatory Bowel Diseases: A Review. Dig. Dis. Sci. 2015, 60, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprong, R.C.; Schonewille, A.J.; van der Meer, R. Dietary Cheese Whey Protein Protects Rats against Mild Dextran Sulfate Sodium–Induced Colitis: Role of Mucin and Microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Karamzin, A.M.; Ropot, A.V.; Sergeyev, O.V.; Khalturina, E.O. Akkermansia muciniphila and Host Interaction within the Intestinal Tract. Anaerobe 2021, 72, 102472. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and Applications of Probiotic Lactobacillus Strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Vigsnæs, L.K.; Brynskov, J.; Steenholdt, C.; Wilcks, A.; Licht, T.R. Gram-Negative Bacteria Account for Main Differences between Faecal Microbiota from Patients with Ulcerative Colitis and Healthy Controls. Benef. Microbes 2012, 3, 287–297. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Lu, Z.; Yang, Y.; Xia, Y.; Lai, P.F.-H.; Ai, L. The Ameliorative Effect of a Lactobacillus Strain with Good Adhesion Ability against Dextran Sulfate Sodium-Induced Murine Colitis. Food Funct. 2019, 10, 397–409. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin Modulates Cecal and Fecal Microbiota Differently Based on Diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Wu, L.; Lyu, Y.; Srinivasagan, R.; Wu, J.; Ojo, B.; Tang, M.; El-Rassi, G.D.; Metzinger, K.; Smith, B.J.; Lucas, E.A.; et al. Astaxanthin-Shifted Gut Microbiota Is Associated with Inflammation and Metabolic Homeostasis in Mice. J. Nutr. 2020, 150, 2687–2698. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Zheng, C. The Emerging Roles of NOD-like Receptors in Antiviral Innate Immune Signaling Pathways. Int. J. Biol. Macromol. 2021, 169, 407–413. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, X.; Zhou, W.; Xu, W.; Dai, Z.; Yan, Y.; Mi, J.; Sun, Y.; Zeng, X.; Cao, Y.; Lu, L. Zeaxanthin Dipalmitate-Enriched Emulsion Stabilized with Whey Protein Isolate-Gum Arabic Maillard Conjugate Improves Gut Microbiota and Inflammation of Colitis Mice. Foods 2022, 11, 3670. https://doi.org/10.3390/foods11223670
Kan X, Zhou W, Xu W, Dai Z, Yan Y, Mi J, Sun Y, Zeng X, Cao Y, Lu L. Zeaxanthin Dipalmitate-Enriched Emulsion Stabilized with Whey Protein Isolate-Gum Arabic Maillard Conjugate Improves Gut Microbiota and Inflammation of Colitis Mice. Foods. 2022; 11(22):3670. https://doi.org/10.3390/foods11223670
Chicago/Turabian StyleKan, Xuhui, Wangting Zhou, Weiqi Xu, Zhuqing Dai, Yamei Yan, Jia Mi, Yi Sun, Xiaoxiong Zeng, Youlong Cao, and Lu Lu. 2022. "Zeaxanthin Dipalmitate-Enriched Emulsion Stabilized with Whey Protein Isolate-Gum Arabic Maillard Conjugate Improves Gut Microbiota and Inflammation of Colitis Mice" Foods 11, no. 22: 3670. https://doi.org/10.3390/foods11223670