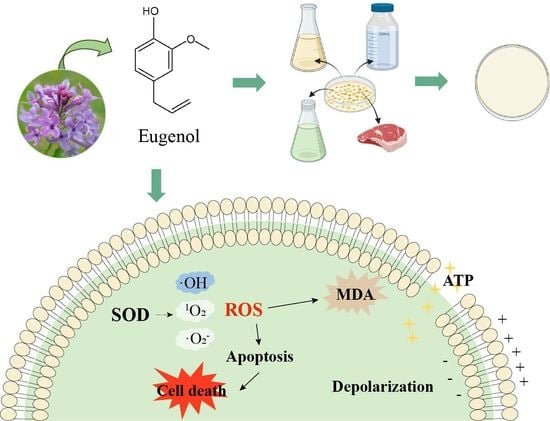
Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Culture Conditions
2.3. Determinations of MIC and MBC
2.4. Growth Curve Analysis
2.5. Inactivation Effect of Eugenol on Sh. flexneri in LB and PBS
2.6. The Bactericidal Effect of Eugenol on Food Easily Contaminated by Sh. flexneri
2.6.1. Inactivation Effect of Eugenol on Sh. flexneri in Vegetable Juice
2.6.2. Inactivation Effect of Eugenol on Sh. flexneri in Minced Pork
2.7. Mechanism of Antibacterial Effects
2.7.1. Assays of ATP Concentrations
2.7.2. Measurement of Membrane Potential
2.7.3. Flow Cytometry Investigation
2.7.4. Effect of Eugenol on Intracellular ROS Production
2.7.5. SOD Activity
2.7.6. Determination of Lipid Peroxidation
2.7.7. Field Emission Scanning Electron Microscope (FESEM) Observations
2.8. Statistical Analysis
3. Results
3.1. MIC and MBC of Eugenol
3.2. Effects of Eugenol on Sh. flexneri Growth Curve
3.3. Inactivation Effect of Eugenol on Sh. flexneri in LB and PBS
3.4. The Bactericidal Effect of Eugenol on Food Easily Contaminated by Sh. flexneri
3.4.1. Inactivation Effect of Eugenol on Sh. flexneri in Vegetable Juice
3.4.2. Inactivation Effect of Eugenol on Sh. flexneri in Minced Pork
3.5. Mechanism of Antibacterial Effects
3.5.1. Effects of Eugenol on Intracellular ATP Concentration of Sh. flexneri
3.5.2. Effects of Eugenol on Cell Membrane Potential of Sh. flexneri
3.5.3. Effects of Eugenol on Cell Membrane Integrity of Sh. flexneri
3.5.4. Effects of Eugenol on ROS Level in Sh. flexneri
3.5.5. Effect of Eugenol on the Activity of SOD of Sh. flexneri
3.5.6. Effect of Eugenol on the Intracellular MDA of Sh. flexneri
3.5.7. Effect of Eugenol on the Cell Morphology of Sh. flexneri
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Q.; Wu, J.; Dai, Z.; Wang, Y. Naked-eyes detection of Shigella flexneri in food samples based on a novel gold nanoparticle-based colorimetric aptasensor. Food Control 2019, 98, 333–341. [Google Scholar] [CrossRef]
- Song, M.S.; Sekhon, S.S.; Shin, W.R.; Kim, H.C.; Min, J.; Ahn, J.Y.; Kim, Y.H. Detecting and discriminating Shigella sonnei using an aptamer-based fluorescent biosensor platform. Molecules 2017, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.; Rodrigues, S.P.; McCormick, B.A. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: Role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect. Immun. 2002, 70, 1150–1158. [Google Scholar] [CrossRef]
- Farzam, N.; Ramon-Saraf, R.; Banet-Levi, Y.; Lerner-Geva, L.; Ashkenazi, S.; Kubler-Kielb, J.; Vinogradov, E.; Robbins, J.B.; Schneerson, R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 2017, 35, 4990–4996. [Google Scholar] [CrossRef]
- Shen, H.; Chen, J.; Xu, Y.; Lai, Z.; Zhang, J.; Yang, H.; Li, Y.; Jiang, M.; Ye, Y.; Bai, X. An outbreak of shigellosis in a Children Welfare Institute caused by a multiple-antibiotic-resistant strain of Shigella flexneri 2a. J. Infect. Public Health 2017, 10, 814–818. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Acid tolerance of Shigella sonnei and Shigella flexneri. J. Appl. Microbiol. 2002, 93, 479–486. [Google Scholar] [CrossRef]
- Tetteh, G.L.; Beuchat, L.R. Exposure of Shigella flexneri to acid stress and heat shock enhances acid tolerance. Food Microbiol. 2003, 20, 179–185. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Liu, C. Control effect of microwave sterilization on soil pathogenic microbe. J. Agric. Sci.-Camb. 2010, 12, 92–94. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, K.H.; Jeoung, T.G.; Kim, S.J.; Cho, S.Y. Sterilization of pathogenic bacteria using titanium dioxide photocatalyst. In Proceedings of the 2006 AIChE Annual Meeting, San Francisco, CA, USA, 11–17 December 2006. [Google Scholar]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef]
- Vaughn, R.H.; Stewart, G.F. Antibiotics as food preservatives. JAMA-J. Am. Med. Assoc. 1960, 174, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Russell, A. Mechanisms of bacterial resistance to non-antibiotics: Food additives and food and pharmaceutical preservatives. J. Appl. Bacteriol. 1991, 71, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Jiao, X.; Zhou, X.; Yang, Z.; Pan, Z.; Huang, J.; Zhou, L.; Qian, X. Systematic functional pandemic strain–specific genes, three genomic islands, two t3sss in foodborne, and clinical Vibrio parahaemolyticus isolates in China. Foodborne Pathog. Dis. 2009, 6, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Thayer, D.W. Irradiation of food—Helping to ensure food safety. N. Engl. J. Med. 2004, 350, 1811–1812. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Cronobacter sakazakii reduction by blueberry proanthocyanidins. Food Microbiol. 2014, 39, 127–131. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Misharina, T.; Samusenko, A. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Matan, N. Growth inhibition of Aspergillus niger by cinnamaldehyde and eugenol. WJST 2007, 4, 41–51. Available online: https://103.58.148.28/index.php/wjst/article/view/124 (accessed on 25 July 2022).
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; Approved Standard M7-A10; LSI: Wayne, PA, USA, 2018. [Google Scholar]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Minhasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol. 2016, 120, 630–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Ji, Y.; Li, W.; Luo, J.; Wang, F.; Zhang, X.; Niu, Z.; Zhou, L.; Yan, L. Endophytic Fungi from Dalbergia odorifera T. Chen Producing Naringenin Inhibit the Growth of Staphylococcus aureus by Interfering with Cell Membrane, DNA, and Protein. J. Med. Food 2021, 24, 116–123. [Google Scholar] [CrossRef]
- Yuan, W.; Teo, C.H.M.; Yuk, H.G. Combined antibacterial activities of essential oil compounds against Escherichia coli O157: H7 and their application potential on fresh-cut lettuce. Food Control 2019, 96, 112–118. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Fennel essential oil loaded porous starch-based microencapsulation as an efficient delivery system for the quality improvement of ground pork. Int. J. Biol. Macromol. 2021, 172, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, S.; Li, J.; Bai, F.; Yang, Y.; Xu, Y.; Liang, S.; Xia, X.; Wang, X.; Shi, C. The antimicrobial activity of coenzyme Q0 against planktonic and biofilm forms of Cronobacter sakazakii. Food Microbiol. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.C.; Motts, S.D.; Towery, P.; Hobson, T.; Harrell, G.; Meurer, L.; Lancaster, K. Inactivation of Salmonella on tainted foods: Using blue light to disinfect cucumbers and processed meat products. Food Sci. Nutr. 2016, 4, 878–887. [Google Scholar] [CrossRef]
- Wang, H.; Niu, Y.; Pan, J.; Li, Q.; Lu, R. Antibacterial effects of Lactobacillus acidophilus surface-layer protein in combination with nisin against Staphylococcus aureus. Lwt-Food Sci. Technol. 2020, 124, 109208. [Google Scholar] [CrossRef]
- Li, R.; Lu, J.; Duan, H.; Tang, C. Biofilm inhibition and mode of action of epigallocatechin gallate against Vibrio mimicus. Food Control 2020, 113, 107148. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, R.; Zhu, C.; Liang, X.; Ettelaie, R.; Jiang, L.; Lin, S. On the mechanism behind enhanced antibacterial activity of alkyl gallate esters against foodborne pathogens and its application in Chinese icefish preservation. Food Microbiol. 2021, 99, 103817. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, Y.F.; Wei, H.; Li, X.X.; Zheng, H.T.; Dong, X.Y.; Xu, T.F.; Meng, J.F. Inhibition efficiency of wood vinegar on grey mould of table grapes. Food Biosci. 2020, 38, 100755. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, S.S.; Kang, D.H. Inactivation dynamics of 222 nm krypton-chlorine excilamp irradiation on Gram-positive and Gram-negative foodborne pathogenic bacteria. Food Res. Int. 2018, 109, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Liu, Y. Ferulic acid inactivates Shigella flexneri through cell membrane destruction, biofilm retardation, and altered gene expression. J. Agric. Food. Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef] [PubMed]
- Van, O.I.; Bagamboula, C.F.; Theys, T.; Vanmuysen, S.C.M.; Michiels, C.W. Inactivation of Escherichia coli and Shigella in acidic fruit and vegetable juices by peroxidase systems. J. Appl. Microbiol. 2006, 101, 242–250. [Google Scholar] [CrossRef]
- Pakbin, B.; Amani, Z.; Allahyari, S.; Mousavi, S.; Mahmoudi, R.; Brück, W.M.; Peymani, A. Genetic diversity and antibiotic resistance of Shigella spp. isolates from food products. Food Sci. Nutr. 2021, 9, 6362–6371. [Google Scholar] [CrossRef] [PubMed]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.W.H.; Park, S.H.; Ha, S.D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crop. Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Shukla, S.; Aziz, F.; Chauhan, A.K.; Ansari, M.B.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Antimicrobial potential of the food-grade additive carvacrol against uropathogenic E. coli based on membrane depolarization, reactive oxygen species generation, and molecular docking analysis. Microb. Pathog. 2020, 142, 104046. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free. Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Kennel, K.B.; Greten, F.R. Immune cell-produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef]
- Liao, X.; Xuan, X.; Li, J.; Suo, Y.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Bactericidal action of slightly acidic electrolyzed water against Escherichia coli and Staphylococcus aureus via multiple cell targets. Food Control 2017, 79, 380–385. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damge. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of intracellular porphyrin, reactive oxygen species, and fatty acids profiles during inactivation of methicillin-resistant Staphylococcus aureus by antimicrobial blue light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, L.; Qu, Y.; Li, H.; Shi, B. Antibacterial activity of buckwheat honey added with ferrous lactate against Pseudomonas aeruginosa. LWT-Food Sci. Technol. 2020, 117, 108624. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, Y.; Wu, Q.; Guo, W.; Chen, H.; Zhang, W.; Li, Y.; Lu, Y.; Wu, Q.; Pan, W.; et al. Antibacterial effect and mechanism against Escherichia coli of polysaccharides from Armillariella tabescens mycelia. Int. J. Biol. Macromol. 2022, 207, 750–759. [Google Scholar] [CrossRef]
- Kang, S.; Kong, F.; Shi, X.; Han, H.; Li, M.; Guan, B.; Yang, M.; Cao, X.; Tao, D.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 2020, 108, 106876. [Google Scholar] [CrossRef]
- Jeon, M.J.; Ha, J.W. Bactericidal and synergistic effects of X-ray irradiation and gallic acid against foodborne pathogens on lettuce. Food Microbiol. 2020, 92, 103584. [Google Scholar] [CrossRef]
- Dias, C.; Nylandsted, J. Plasma membrane integrity in health and disease: Significance and therapeutic potential. Cell Discov. 2021, 7, 4. [Google Scholar] [CrossRef]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef]
- Mempin, R.; Tran, H.; Chen, C.; Gong, H.; Ho, K.K.; Lu, S. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013, 13, 301. [Google Scholar] [CrossRef]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Sánchez, E.; García, S.; Heredia, N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wang, Q.Y.; Ren, E.F.; Zeng, X.A.; Wang, L.H.; He, T.F.; Wen, Q.H.; Brennan, C.S. Multi-target antibacterial mechanism of eugenol and its combined inactivation with pulsed electric fields in a hurdle strategy on Escherichia coli. Food Control 2019, 106, 106742. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism. Foods 2022, 11, 2565. https://doi.org/10.3390/foods11172565
Bai X, Li X, Liu X, Xing Z, Su R, Wang Y, Xia X, Shi C. Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism. Foods. 2022; 11(17):2565. https://doi.org/10.3390/foods11172565
Chicago/Turabian StyleBai, Xiangyang, Xuejiao Li, Xue Liu, Zeyu Xing, Ruiying Su, Yutang Wang, Xiaodong Xia, and Chao Shi. 2022. "Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism" Foods 11, no. 17: 2565. https://doi.org/10.3390/foods11172565