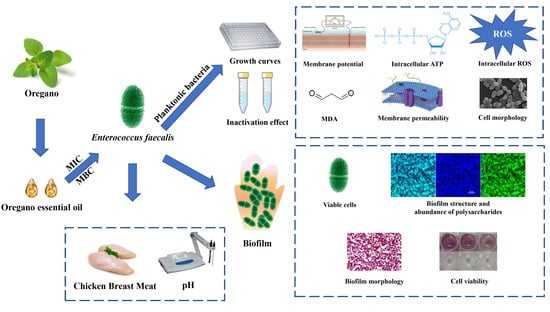
The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus faecalis and Its Application in Chicken Breast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Growth Conditions
2.3. MIC and MBC Determinations
2.4. Growth Curves
2.5. Inactivation Effect of OEO against E. faecalis in BHI Broth and PBS
2.6. Mechanism of Antibacterial Effects
2.6.1. Membrane Potential
2.6.2. Intracellular Adenosine Triphosphate (ATP)
2.6.3. Intracellular Reactive Oxygen Species (ROS)
2.6.4. Extracellular Malondialdehyde (MDA)
2.6.5. Bacterial Morphology
2.6.6. Membrane Integrity
2.7. Inactivation Effect of OEO against E. faecalis Biofilms
2.7.1. Biofilm Formation
2.7.2. Viable Cell Enumeration
2.7.3. Confocal Laser Scanning Microscopy (CLSM) Observations
2.7.4. Optical Microscope-Based Observation and Quantitation
2.7.5. Detection of the Viability of Cells in the Biofilm
2.8. Application of OEO in Raw Chicken Breast Meat during Storage at 10 °C
2.8.1. Antimicrobial Effect of OEO on Inoculated E. faecalis in Raw Chicken Breast Meat
2.8.2. pH Assay
2.9. Statistical Analysis
3. Results
3.1. MICs and MBCs
3.2. Growth Curves
3.3. Antimicrobial Effect of OEO on E. faecalis
3.4. Antibacterial Mechanism of OEO against E. faecalis
3.4.1. Membrane Potential
3.4.2. Intracellular ATP
3.4.3. Intracellular ROS
3.4.4. Extracellular MDA
3.4.5. Membrane Integrity
3.4.6. Cell Morphology
3.5. Inactivation of OEO on E. faecalis in Biofilms
3.5.1. Viable Cell Enumeration
3.5.2. Biofilm Morphology
3.5.3. CLSM Observations
3.5.4. Effect of OEO on Cell Viability in Biofilm
3.6. Application of the OEO to Raw Chicken Breast Meat
3.6.1. Antimicrobial Activity
3.6.2. pH Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Hu, Z.; Du, L.; Liu, F.; Yuan, K. Inhibition of Enterococcus faecalis Growth and Cell Membrane Integrity by Perilla frutescens Essential Oil. Foodborne Pathog. Dis. 2020, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Virulence factors of Enterococcus spp. presented in food. LWT 2017, 75, 670–676. [Google Scholar] [CrossRef]
- Giraffa, G. Enterococci from foods. FEMS Microbiol. Rev. 2002, 24, 163–171. [Google Scholar] [CrossRef] [PubMed]
- McGowan-Spicer, L.L.; Fedorka-Cray, P.J.; Frye, J.G.; Meinersmann, R.J.; Barrett, J.B.; Jackson, C.R. Antimicrobial resistance and virulence of Enterococcus faecalis isolated from retail food. J. Food Prot. 2008, 71, 760–769. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, H.W.; Park, S.M.; Seo, G.H.; Cho, T.J.; Yu, H.R.; Rhee, M.S. Virulence patterns and prevalence of seven Enterococcus species isolated from meats and leafy vegetables in South Korea. Food Control 2020, 108, 106867. [Google Scholar] [CrossRef]
- Tan, L.; Li, H.; Chen, B.; Huang, J.; Li, Y.; Zheng, H.; Wang, J.J. Dual-species biofilms formation of Vibrio parahaemolyticus and Shewanella putrefaciens and their tolerance to photodynamic inactivation. Food Control 2021, 125, 107983. [Google Scholar] [CrossRef]
- Liu, F.; Du, L.; Zhao, T.; Zhao, P.; Doyle, M.P. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control 2017, 78, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Fallah, F.; Yousefi, M.; Pourmand, M.R.; Hashemi, A.; Alam, A.N.; Afshar, D. Phenotypic and genotypic study of biofilm formation in Enterococci isolated from urinary tract infections. Microb. Pathog. 2017, 108, 85–90. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-Lopez, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2172–2191. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; do Prado, I.N.; Machinski, M.; de Abreu Filho, B.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Janani, K.; Ajitha, P.; Sandhya, R.; Teja, K.V.; Secondary, J.K.; Ajitha, P.; Sandhya, R.; Teja, K.V.; Tertiary, J.K.; Ajitha, P.; et al. Chemical constituent, minimal inhibitory concentration, and antimicrobial efficiency of essential oil from oreganum vulgare against Enterococcus faecalis: An in vitro study. J. Conserv. Dent. 2019, 22, 538–543. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L.; Secondary, M.A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L.; et al. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.D.; Amaral, A.F.; Schaefer, G.; Luciano, F.B.; de Andrade, C.; Costa, L.B.; Rostagno, M.H. Antimicrobial effect against different bacterial strains and bacterial adaptation to essential oils used as feed additives. Can. J. Vet. Res. 2015, 79, 285–289. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; Approved Standard M7-A10; LSI: Wayne, PA, USA, 2018. [Google Scholar]
- Shi, C.; Sun, Y.; Liu, Z.Y.; Guo, D.; Sun, H.H.; Sun, Z.; Xia, X.D. Inhibition of Cronobacter sakazakii Virulence Factors by Citral. Sci Rep. 2017, 7, 43243. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Xia, X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, E.; Garcia, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2021, 76, 6888–6894. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.J.; Ha, J.W. Bactericidal and synergistic effects of X-ray irradiation and gallic acid against foodborne pathogens on lettuce. Food Microbiol. 2020, 92, 103584. [Google Scholar] [CrossRef]
- Shao, P.; Wang, P.; Niu, B.; Kang, J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018, 119, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2013, 238, 589–596. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalaradjou, M.A.; Venkitanarayanan, K. Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Prot. 2011, 74, 200–208. [Google Scholar] [CrossRef]
- Peng, G.; Hou, X.; Zhang, W.; Song, M.; Yin, M.; Wang, J.; Li, G. Alkyl rhamnosides, a series of amphiphilic materials exerting broad-spectrum anti-biofilm activity against pathogenic bacteria via multiple mechanisms. Artif. Cells Nanomed. Biotechnol. 2018, 46, S217–S232. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, H.; Guo, X.; Guo, D.; Yang, Y.; Shi, C. Antibiofilm activity of shikonin against Listeria monocytogenes and inhibition of key virulence factors. Food Control 2021, 120, 107558. [Google Scholar] [CrossRef]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.K. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zheng, P.; Tian, F.; Zhang, Z.; Sun, Z.; Cai, L. Development of edible composite film based on chitosan nanoparticles and their application in packaging of fresh red sea bream fillets. Food Control 2022, 132, 108545. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Gong, H.; Sun, Z.; Du, L.; Wang, D. Antibacterial and antibiofilm activities of thyme oil against foodborne multiple antibiotics-resistant Enterococcus faecalis. Poult. Sci. 2020, 99, 5127–5136. [Google Scholar] [CrossRef]
- Silva, S.; Alves, N.; Silva, P.; Vieira, T.; Maciel, P.; Castellano, L.R.; Albuquerque, D. Antibacterial Activity of Rosmarinus officinalis, Zingiber officinale, Citrus aurantium bergamia, and Copaifera officinalis Alone and in Combination with Calcium Hydroxide against Enterococcus faecalis. BioMed Res. Int. 2019, 2019, 8129439. [Google Scholar] [CrossRef] [Green Version]
- Bot, C.; Prodan, C. Probing the membrane potential of living cells by dielectric spectroscopy. Eur. Biophys. J. 2009, 38, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.A.A.; Cheung, B.P.K.; Matinlinna, J.; Levesque, C.M.; Neelakantan, P. Trans.-cinnamaldehyde potently kills Enterococcus faecalis biofilm cells and prevents biofilm recovery. Microb. Pathog. 2020, 149, 104482. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural Plant-Derived Chemical Compounds as Listeria monocytogenes Inhibitors In Vitro and in Food Model Systems. Pathogens 2020, 10, 12. [Google Scholar] [CrossRef]
- Plasek, J.; Gaskova, D.; Ludwig, J.; Hofer, M. Early changes in membrane potential of Saccharomyces cerevisiae induced by varying extracellular K(+), Na (+) or H (+) concentrations. J. Bioenerg. Biomembr. 2013, 45, 561–568. [Google Scholar] [CrossRef]
- Mempin, R.; Tran, H.; Chen, C.N.; Gong, H.; Ho, K.K.; Lu, S.W. Release of extracellular ATP by bacteria during growth. BMC Microbiol. 2013, 13, 301. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, K.; Zhong, Q.X. Eugenol Nanoencapsulated by Sodium Caseinate: Physical, Antimicrobial, and Biophysical Properties. Food Biophys. 2018, 13, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.Y.; Zhao, C.T.; Lin, L. Antibacterial Activity of Helichrysum italicum Oil on Vegetables and Its Mechanism of Action. J. Food Process. Preserv. 2015, 39, 2663–2672. [Google Scholar] [CrossRef]
- Shi, Y.G.; Zhang, R.R.; Zhu, C.M.; Xu, M.F.; Gu, Q.; Ettelaie, R.; Leng, X.Y. Antimicrobial mechanism of alkyl gallates against Escherichia coli and Staphylococcus aureus and its combined effect with electrospun nanofibers on Chinese Taihu icefish preservation. Food Chem. 2021, 346, 128949. [Google Scholar] [CrossRef]
- Li, C.Z.; Zhang, C.H.; Chen, X.C.; Cui, H.Y.; Lin, L. The Interference Mechanism of Basil Essential Oil on the Cell Membrane Barrier and Respiratory Metabolism of Listeria monocytogenes. Front. Microbiol. 2022, 13, 855905. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS Defense Systems and Terminal Oxidases in Bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.X.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Andrade, N.J.; Ismail, F.M.D.; Barbosa, M.C.A. Exposure to Anacardiaceae Volatile Oils and Their Constituents Induces Lipid Peroxidation within Food-Borne Bacteria Cells. Molecules 2012, 17, 9728–9740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Lee, D.G. Resveratrol induces membrane and DNA disruption via pro-oxidant activity against Salmonella typhimurium. Biochem. Biophys. Res. Commun. 2017, 489, 228–234. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.Y.; Liu, R.J.; Zhang, D.; Wang, X.; Sun, R.C.; Gong, G.L. Antibacterial mechanism of thymol against Enterobacter sakazakii. Food Control 2021, 123, 107716. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Becerril, R.; Manso, S.; Nerin, C.; Gómez-Lus, R. Antimicrobial activity of Lauroyl Arginate Ethyl (LAE), against selected food-borne bacteria. Food Control 2013, 32, 404–408. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Bai, J.R.; Zhong, K.; Wu, Y.P.; Elena, G.; Gao, H. Antibiofilm activity of shikimic acid against Staphylococcus aureus. Food Control 2019, 95, 327–333. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yin, Y.; Guo, D.; Jin, T.; Guan, N.; Shi, C. Antibiofilm activity of coenzyme Q0 against Salmonella Typhimurium and its effect on adhesion-invasion and survival-replication. Appl. Microbiol. Biotechnol. 2019, 103, 8545–8557. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2015, 13, 20–26. [Google Scholar] [CrossRef]
- Guo, D.; Wang, S.; Li, J.; Bai, F.; Yang, Y.; Xu, Y.; Shi, C. The antimicrobial activity of coenzyme Q0 against planktonic and biofilm forms of Cronobacter sakazakii. Food Microbiol. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.; Wang, F.; Liu, Y.; Zhu, Y.; Du, L.; Xu, W. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2020, 86, 103344. [Google Scholar] [CrossRef]
- Costa, G.A.; Rossatto, F.C.P.; Medeiros, A.W.; Correa, A.P.F.; Brandelli, A.; Frazzon, A.P.G.; Da Motta, A.D. Evaluation antibacterial and antibiofilm activity of the antimicrobial peptide P34 against Staphylococcus aureus and Enterococcus faecalis. An. Acad. Bras. Cienc. 2018, 90, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, D.A.C.; da Silva, A.F.; Negri, M.; de Abreu, B.A.; Machinski, M.; Patussi, E.V.; Mikcha, J.M.G. Antibacterial and antibiofilm activity of carvacrol against Salmonella enterica serotype Typhimurium. J. Pharm. Sci. 2018, 54, e17229. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Ducci, B.; Magnanini, A.; Lo Nostro, A. Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol. 2014, 41, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.; Li, Y.; Xu, H.; Han, X.; Wang, R.; Shi, C. Antimicrobial Activity and Antibiofilm Potential of Coenzyme Q0 against Salmonella Typhimurium. Foods 2021, 10, 1211. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, P.; He, Y.; Kang, S.; Shen, C.; Wang, S.; Shi, C. Antibacterial Effect of Oregano Essential Oil against Vibrio vulnificus and Its Mechanism. Foods 2022, 11, 403. [Google Scholar] [CrossRef]
- Hayat, M.N.; Kaka, U.; Sazili, A.Q. Assessment of Physicochemical Characteristics and Microbiological Quality in Broiler Chicken Breast Muscle (Pectoralis major) Subjected to Different Temperatures and Lengths of Cold Transportation. Foods 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Effect of Ferulic Acid-Grafted-Chitosan Coating on the Quality of Pork during Refrigerated Storage. Foods 2021, 10, 1374. [Google Scholar] [CrossRef] [PubMed]













| Strain | Origin | MIC(μL/mL) | MBC(μL/mL) |
|---|---|---|---|
| ATCC 29212 | Urine | 0.25 | 0.50 |
| SX20FC004 | Chicken cloacal swab | 0.50 | 1.00 |
| SX20FC006 | Chicken cloacal swab | 0.50 | 0.50 |
| SX20FC181 | Pig nasal swab | 0.50 | 0.50 |
| SX20FC182 | Pig nasal swab | 0.50 | 0.50 |
| SX20FC318 | Bovine breast swab | 0.50 | 1.00 |
| SX20FC319 | Bovine breast swab | 0.25 | 0.50 |
| SX20FCN4 | Raw milk | 0.50 | 0.50 |
| SX20FCN6 | Raw milk | 0.50 | 0.50 |
| SX20FCN9 | Raw milk | 0.25 | 0.50 |
| Storage Time (Hour) | 0 | 3 | 12 | 24 |
|---|---|---|---|---|
| Control | 6.15 ± 0.01 cC | 6.18 ± 0.03 cC | 6.35 ± 0.01 abA | 6.31 ± 0.01 aB |
| 10 MIC | 6.18 ± 0.01 bD | 6.21 ± 0.01b cC | 6.33 ± 0.01 bA | 6.32 ± 0.01 aB |
| 20 MIC | 6.20 ± 0.01a bC | 6.28 ± 0.01 aA | 6.27 ± 0.01 cA | 6.23 ± 0.01 bB |
| 30 MIC | 6.21 ± 0.01a bC | 6.26 ± 0.02 aB | 6.36 ± 0.02 aA | 6.23 ± 0.01 bC |
| 40 MIC | 6.21 ± 0.02 aB | 6.22 ± 0.01 bB | 6.33 ± 0.02 bA | 6.21 ± 0.01 cB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Tan, Y.; Lv, Y.; Fang, J.; Zhou, Y.; Gao, X.; Zhu, H.; Shi, C. The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus faecalis and Its Application in Chicken Breast. Foods 2022, 11, 2296. https://doi.org/10.3390/foods11152296
Zhan X, Tan Y, Lv Y, Fang J, Zhou Y, Gao X, Zhu H, Shi C. The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus faecalis and Its Application in Chicken Breast. Foods. 2022; 11(15):2296. https://doi.org/10.3390/foods11152296
Chicago/Turabian StyleZhan, Xiangjun, Yingzhu Tan, Yingmei Lv, Jianing Fang, Yuanjian Zhou, Xing Gao, Huimin Zhu, and Chao Shi. 2022. "The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus faecalis and Its Application in Chicken Breast" Foods 11, no. 15: 2296. https://doi.org/10.3390/foods11152296