Traditional Fermented Foods from Ecuador: A Review with a Focus on Microbial Diversity
Abstract
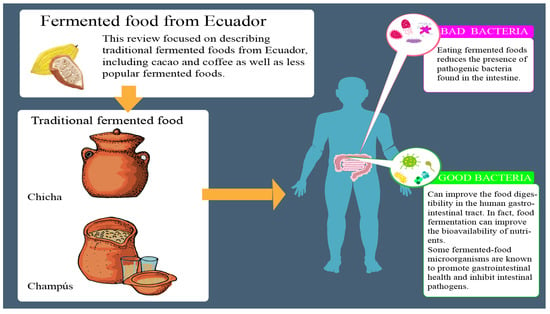
:1. Introduction
2. Fermented Foods from Ecuador
2.1. Cacao
2.2. Coffee
2.3. Traditional Fermented Beverages
2.3.1. Chicha
2.3.2. Champús
3. Functionality of Microorganisms
| Community | Species | Fermented Food * | Study Observations | Reference |
|---|---|---|---|---|
| Yeast | Candida californica | C, CJ | The genus Candida is frequently found in spontaneous fermentation processes and has been assessed as a starter culture for alcohol production. | [67] |
| Candida humilis | CJ, Cf | |||
| Candida quercitrusa | Cf | |||
| Candida sake | CJ, CM | |||
| Candida solani | C, CJ | |||
| Candida sorbosivorans | C | |||
| Candida sorboxylosa | CJ | |||
| Candida zeylanoides | CJ | |||
| Candida vinaria | CJ | |||
| Candida tropicalis | C, CY | Used as a starter culture in sorghum beer and barley malt medium. | [79,80] | |
| Dekkera anomala | CJ | These species were related to the production of unpleasant aromas and were not recommended as starter culture. | [81] | |
| Dekkera bruxellensis | CJ, SC | |||
| Hanseniaspora opuntiae | C, CY | Was used as a possible starter culture in cacao fermentation. | [82] | |
| Hanseniaspora uvarum | Cf | Starter culture for the production of volatile compounds in fermented foods and beverages. | [71,83] | |
| Hanseniaspora spp. | CJ, CH, Cf | |||
| Issatchenkia orientalis | CH | Malic acid reduction and interaction mechanisms with S. cerevisiae. | [84] | |
| Kazachstania exigua | CJ | Starter culture for cacao fermentation. | [85] | |
| Kodamaea ohmeri | CY | |||
| Kluyveromyces marxianus | C | |||
| Pichia fermentans | CJ, CM, CH | Starter culture for wine to stabilize color and increase fruit and floral aromas. | [86] | |
| Pichia kluyveri | C, CJ, CH | Starter culture to increases volatile thiols (3-mercaptohexanol and its acetylated derivative 3-mercaptohexyl acetate) with fruity aroma as passion fruit and grapefruit. | [87] | |
| Pichia kudriavzevii | C, | Starter culture for cacao fermentation. | [85] | |
| Pichia manshurica | SC | |||
| Rhodotorula minuta | C | [60] | ||
| Rhodotorula mucilaginosa | CJ, Cf | |||
| Saccharomyces cerevisiae | C, Cf, CJ, SC, CY, CH | Starter culture for different types of beer. | [88] | |
| Saccharomycodes ludwigii | CJ, CM | |||
| Torulospora delbrueckii | C, CJ, CM, CY, CH | Alcohol production to improve flavor diversity. | [60,89] | |
| Zygoascus hellenicus | CJ | |||
| Zygosaccharomyces fermentati | CH | |||
| LAB | Enterococcus casseliflavus | C | Food bio-preservative. | [90] |
| Enterococcus saccharolyticus | C | |||
| Enterococcus sp. | C | |||
| Fructobacillus durionis | C | Some strains with probiotic potential. | [49] | |
| Fructobacillus ficulneus | C | |||
| Fructobacillus tropaeoli | C | |||
| Lactobacillus acidophilus | C, CY | Starter cultures for steering food fermentation processes. Some specific strains have probiotic potential. | [91,92]. | |
| Lactobacillus amylovorus | C | |||
| Levilactobacillus brevis | C, Cf | |||
| Liquorilactobacillus cacaonum | C | |||
| Lacticaseibacillus casei | C, CJ | |||
| Loigolactobacillus coryniformis | C | |||
| Lactobacillus delbrueckii | C, CY | |||
| Lactiplantibacillus fabifermentans | C | |||
| Lentilactobacilluss farraginis | C | |||
| Licmosilactobacillus fermentum | C, CY | |||
| Lactobacillus garvieae | C | |||
| Liquorilactobacillus nagelii | C | |||
| Lactiplantibacillus plantarum | C, O | Starter culture in different fermented foods and beverages. | [31,93] | |
| Limosilactobacillus reuteri | CY | Starter culture can produce antimicrobial molecules, such as organic acids, ethanol, and reuterin. | [94] | |
| Lactobacillus delbruckii subsp. Lactis | C, CY, Cf | Starter culture in yoghurt. | [95] | |
| Lactococcus hircilactis | Cf | |||
| Leuconostoc fallax | C, Cf | Starter culture for the production of butyric acid. | [96] | |
| Leuconostoc mesenteroides | C, CY, O | |||
| Leuconostoc pseudomesenteroides | C, Cf | |||
| Streptococcus thermopjhilus | CY | Starter culture in yoghurt and cheese by its rapidly growing in low pH conditions. | [97] | |
| Streptococcus salivarius | O | |||
| Weissella cibaria | C | High presence in different fermented foods; its redox potential influences the aromatic profile. Qualified Presumption of Safety (QPS) for food applications in in process. | [77] | |
| Weissella fabaria | C | |||
| Weissella confusa | O | |||
| AAB | Acetobacter cibinongensis | C, Cf | Starter culture for food fermentation processes to favor the production of volatile compounds. | [98] |
| Acetobacter lovaniensis | C | |||
| Acetobacter malorum/cerevisiae | C | |||
| Acetobacter malorum/indonesiensis | C, Cf | |||
| Acetobacter fabarum | C, Cf | |||
| Acetobacter ghanensis | C | |||
| Acetobacter orientalis | C, Cf | |||
| Acetobacter okinawensis | Cf | |||
| Acetobacter pasteurianus | C | |||
| Acetobacter peroxydans | C | |||
| Acetobacter pomorum | C | |||
| Acetobacter senegalensis | C, Cf | |||
| Acetobacter syzygii | C | |||
| Acetobacter thaillandicus | Cf | |||
| Frateuria aurantia | C | Starter culture potential when high concentrations of glucose are present. | [78] | |
| Microbacterium lacticum | C | |||
| Gluconobacter cerevisiae | Cf | |||
| Gluconobacter oxydans | C | |||
| Gluconobacter sp. | C |
4. Benefits and Risk
5. Future Perspectives
6. Development of Starter Cultures
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ojha, K.S.; Tiwari, B.K. Novel Food Fermentation Technologies BT—Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–5. [Google Scholar]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus paracasei 4341 as adjunct culture to enhance flavor in short ripened Caciotta-type cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; da Cruz PedrozoMiguel, M.G.; Evangelista, S.R.; Martins, P.M.M.; van Mullem, J.; Belizario, M.H.; Schwan, R.F. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef]
- Toktaş, B.; Bildik, F.; Özçelik, B. Effect of fermentation on anthocyanin stability and in vitro bioaccessibility during shalgam (şalgam) beverage production. J. Sci. Food Agric. 2018, 98, 3066–3075. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Aslam, H.; Green, J.; Jacka, F.N.; Collier, F.; Berk, M.; Pasco, J.; Dawson, S.L. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutr. Neurosci. 2020, 23, 659–671. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.-C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.M.; Altuntas, E.G.; Juneja, V.K. Microbial Fermentation in Food Preservation BT—Microbial Control and Food Preservation: Theory and Practice; Juneja, V.K., Dwivedi, H.P., Sofos, J.N., Eds.; Springer: New York, NY, USA, 2017; pp. 281–298. [Google Scholar]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food. Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- IMARC. Global Yogurt Market Strengthened by Growing Consumers’ Health Awareness. 2022. Available online: https://www.imarcgroup.com/global-yogurt-market-strengthened-growing (accessed on 28 April 2022).
- Kim, J.; Adhikari, K. Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- IMARC. Vinegar Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. 2021. Available online: https://www.imarcgroup.com/vinegar-manufacturing-plant (accessed on 28 April 2022).
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, Á. Water kefir, a fermented beverage containing probiotic microorganisms: From ancient and artisanal manufacture to industrialized and regulated commercialization. Future Foods 2022, 5, 100123. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Sánchez, V.H.; Iglesias, C.; Zambrano, J.L. Diagnóstico y prospectiva de la cadena de valor del cacao en América Latina y El Caribe. In La Cadena de Valor del Cacao en América Latina y El Caribe; Sánchez, V.H., Iglesias, C., Zambrano, J.L., Eds.; INIAP: Quito, Ecuador, 2019; p. 104. [Google Scholar]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The use and domestication of Theobroma cacao during the mid-Holocene in the upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented food products in the era of globalization: Tradition meets biotechnology innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Camu, N.; Falony, G.; De Vuyst, L. Comparison of the bacterial species diversity of spontaneous cocoa bean fermentations carried out at selected farms in Ivory Coast and Brazil. Food Microbiol. 2011, 28, 964–973. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hamdouche, Y.; Guehi, T.; Durand, N.; Kedjebo, K.B.D.; Montet, D.; Meile, J.C. Dynamics of microbial ecology during cocoa fermentation and drying: Towards the identification of molecular markers. Food Control 2015, 48, 117–122. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and Biodiversity of Bacterial and Yeast Communities during Fermentation of Cocoa Beans. Appl. Environ. Microbiol. 2018, 84, e01164-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L. Molecular identification and physiological characterization of yeasts, lactic acid bacteria and acetic acid bacteria isolated from heap and box cocoa bean fermentations in West Africa. Int. J. Food Microbiol. 2016, 216, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Alvareza, J.P.; de C. Netoa, D.P.; Soccol, V.T.; Tanobea, V.O.A.; Rogezb, H.; Góes-Netoc, A.; Soccola, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.-M.; De Vuyst, L. Species Diversity, Community Dynamics, and Metabolite Kinetics of the Microbiota Associated with Traditional Ecuadorian Spontaneous Cocoa Bean Fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, V.T.T.; Zhao, J.; Fleet, G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015, 205, 54–67. [Google Scholar] [CrossRef]
- Ouattara, H.D.; Ouattara, H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine—Diacetyl—Desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef]
- Shi, C.; Knøchel, S. Susceptibility of dairy associated molds towards microbial metabolites with focus on the response to diacetyl. Food Control 2021, 121, 107573. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016, 236, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Börner, R.A.; Kandasamy, V.; Axelsen, A.M.; Nielsen, A.T.; Bosma, E.F. High-throughput Genome Editing Tools for Lactic Acid Bacteria: Opportunities for Food, Feed, Pharma and Biotech. FEMS Microbiol. Lett. 2018, 366, 291. [Google Scholar] [CrossRef]
- Illeghems, K.; Weckx, S.; De Vuyst, L. Applying meta-pathway analyses through metagenomics to identify the functional properties of the major bacterial communities of a single spontaneous cocoa bean fermentation process sample. Food Microbiol. 2015, 50, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Moura, F.G.; de Melo Pereira, G.V.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-López, C. Functional Biodiversity of Yeasts Isolated from Colombian Fermented and Dry Cocoa Beans. Microorganisms 2020, 8, 1086. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial diversity associated with spontaneous coffee bean fermentation process and specialty coffee production in northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Slavova, G.; Georgieva, V. World production of coffee imports and exportsin Europe, Bulgaria and USA. Trakia J. Sci. 2019, 17, 619–626. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Consumers’ Perceptions of Coffee Health Benefits and Motives for Coffee Consumption and Purchasing. Nutrients 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Selmar, D.; Bytof, G.; Knopp, S.-E.; Breitenstein, B. Germination of Coffee Seeds and its Significance for Coffee Quality. Plant Biol. 2006, 8, 260–264. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2016, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, S.R.; Silva, C.F.; da CruzMiguel, M.G.P.; de SouzaCordeiro, C.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Dong, H.; Yang, P.; Yang, R.; Lu, J.; Lv, J.; Sheng, J. Culture-Dependent and -Independent Methods to Investigate the Predominant Microorganisms Associated with Wet Processed Coffee. Curr. Microbiol. 2016, 73, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Isolation, Identification, and Characterization of Pectinolytic Yeasts for Starter Culture in Coffee Fermentation. Microorganisms 2019, 7, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, S.J.; Simão, J.B.P.; Pylro, V.S.; Schwan, R.F. The Altitude of Coffee Cultivation Causes Shifts in the Microbial Community Assembly and Biochemical Compounds in Natural Induced Anaerobic Fermentations. Front. Microbiol. 2021, 12, 671395. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Batista, N.N.; da Cruz Pedrozo Miguel, M.G.; Simão, J.B.P.; Soares, J.R.; Schwan, R.F. Coffee growing altitude influences the microbiota, chemical compounds and the quality of fermented coffees. Food Res. Int. 2020, 129, 108872. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; ten Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef]
- Fitri; Tawali, A.B.; Laga, A. Luwak coffee in vitro fermentation: Literature review. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 12096. [Google Scholar] [CrossRef]
- Watanabe, H.; Ng, C.H.; Limviphuvadh, V.; Suzuki, S.; Yamada, T. Gluconobacter dominates the gut microbiome of the Asian palm civet Paradoxurus hermaphroditus that produces kopi luwak. PeerJ 2020, 8, e9579. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J.; Magalhães, F.; Aranda, A.; Matallana, E.; Gibson, B. Potential application of yeasts from Ecuadorian chichas in controlled beer and chicha production. Food Microbiol. 2021, 98, 103644. [Google Scholar] [CrossRef] [PubMed]
- Faria-Oliveira, F.; Diniz, R.; Godoy-Santos, F.; Piló, F.; Mezadri, H.; Castro, I.; Brandão, R. El papel de la levadura y las bacterias del ácido láctico en la producción de bebidas fermentadas en América del Sur. In Food Production and Industry; IntechOpen: London, UK, 2015; pp. 107–135. [Google Scholar]
- Piló, F.B.; Carbajal-Barriga, E.J.; Guamán-Burneo, M.C.; Portero-Barahona, P.; Morato-Dias, A.M.; Daher de Freitas, L.; Oliverira Gomes, F.; Rosa, C.A. Saccharomyces cerevisiae populations and other yeasts associated with indigenous beers (chicha) of Ecuador. Braz. J. Microbiol. 2018, 49, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, G.A.C.; Palma, G.B.A.; Sandoval-Cañas, G.J.; Ordoñez-Araque, R.H. Ancestral fermented indigenous beverages from South America made from cassava (Manihot esculenta). Food Sci. Technol. 2021, 41, 360–367. [Google Scholar] [CrossRef]
- Freire, A.L.; Zapata, S.; Mosquera, J.; Mejia, M.L.; Trueba, G. Bacteria associated with human saliva are major microbial components of Ecuadorian indigenous beers (chicha). PeerJ 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Grijalva-Vallejos, N.; Aranda, A.; Matallana, E. Evaluation of yeasts from Ecuadorian chicha by their performance as starters for alcoholic fermentations in the food industry. Int. J. Food Microbiol. 2020, 317, 108462. [Google Scholar] [CrossRef]
- Colehour, A.M.; Meadow, J.F.; Liebert, M.A.; Cepon-Robins, T.J.; Gildner, T.E.; Urlacher, S.S.; Bohannan, B.J.M.; Snodgrass, J.J.; Sugiyama, L.S. Local domestication of lactic acid bacteria via cassava beer fermentation. PeerJ 2014, 2, e479. [Google Scholar] [CrossRef] [Green Version]
- Resende, L.V.; Pinheiro, L.K.; da Cruz Pedroso Miguel, M.G.; Ramos, C.L.; Vilela, D.M.; Schwan, R.F. Microbial community and physicochemical dynamics during the production of ‘Chicha’, a traditional beverage of Indigenous people of Brazil. World J. Microbiol. Biotechnol. 2018, 34, 46. [Google Scholar] [CrossRef]
- Bassi, D.; Orrù, L.; Cabanillas Vasquez, J.; Cocconcelli, P.S.; Fontana, C. Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms 2020, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.; Lacerda, I.; Libkind, D.; Lopes, C.; Carvajal Barriga, E.; Rosa, C. Traditional foods and beverages from South America: Microbial communities and production strategies. In Industrial Fermentation: Food Processes, Nutrient Sources and Production Strategies; Nova Science Pub Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Osorio-Cadavid, E.; Chaves-López, C.; Tofalo, R.; Paparella, A.; Suzzi, G. Detection and identification of wild yeasts in Champús, a fermented Colombian maize beverage. Food Microbiol. 2008, 25, 771–777. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Sirén, K.; Mak, S.S.T.; Melkonian, C.; Carøe, C.; Swiegers, J.H.; Molenaar, D.; Fischer, U.; Gilbert, M.T.P. Taxonomic and Functional Characterization of the Microbial Community During Spontaneous in vitro Fermentation of Riesling Must. Front. Microbiol. 2019, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Gibson, B. A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Appl. Microbiol. Biotechnol. 2020, 104, 3745–3756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The Interaction between Saccharomyces cerevisiae and Non-Saccharomyces Yeast during Alcoholic Fermentation Is Species and Strain Specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Storti, L.V.; Glibota, N.A.; Parente, E. Aerobic and respirative growth of heterofermentative lactic acid bacteria: A screening study. Food Microbiol. 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2021, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Önning, G.; Palm, R.; Linninge, C.; Larsson, N. New Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus strains: Well tolerated and improve infant microbiota. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Soccol, V.T.; Soccol, C.R. Current state of research on cocoa and coffee fermentations. Curr. Opin. Food Sci. 2016, 7, 50–57. [Google Scholar] [CrossRef]
- Alloue-Boraud, W.A.M.; N’Guessan, K.F.; Djeni, N.T.; Hiligsmann, S.; Djè, K.M.; Delvigne, F. Fermentation profile of Saccharomyces cerevisiae and Candida tropicalis as starter cultures on barley malt medium. J. Food Sci. Technol. 2015, 52, 5236–5242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’Guessan, F.K.; N’Dri, D.Y.; Camara, F.; Djè, M.K. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J. Microbiol. Biotechnol. 2010, 26, 693–699. [Google Scholar] [CrossRef]
- Yokota, K.; Takeo, A.; Abe, H.; Kurokawa, Y.; Hashimoto, M.; Kajimoto, K.; Tanaka, M.; Murayama, S.; Nakajima, Y.; Taniguchi, M.; et al. Application of Micropore Device for Accurate, Easy, and Rapid Discrimination of Saccharomyces pastorianus from Dekkera spp. Biosensors 2021, 11, 272. [Google Scholar] [CrossRef]
- Sin, O.T. Effect of selected yeast starter in cocoa fermentation: A Study on Antioxidant Content, Volatile Organic Compounds and Sensory Profile of Malaysian Cocoa Beans and Chocolates Produced. Ph.D. Thesis, Monash University, Melbourne, Australia, 2021. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Zhang, W.; Weng, P.; Wu, Z. Interaction profile of a mixed-culture fermentation of Issatchenkia orientalis and Saccharomyces cerevisiae by transcriptome sequencing. Br. Food J. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; da Silva Martins, L.H.; Lopes, A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Kong, C.-L.; Li, A.-H.; Su, J.; Wang, X.-C.; Chen, C.-Q.; Tao, Y.-S. Flavor modification of dry red wine from Chinese spine grape by mixed fermentation with Pichia fermentans and S. cerevisiae. LWT 2019, 109, 83–92. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Hutajulu, I.B.E.; Kulla, P.D.K.; Retnaningrum, E. Diversity of lactic acid bacteria isolated during fermentation of indigenous cassava obtained from Sumba, East Nusa Tenggara, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 2561–2570. [Google Scholar] [CrossRef]
- Munanga, B.d.J.C.; Loiseau, G.; Grabulos, J.; Mestres, C. Modeling Lactic Fermentation of Gowé Using Lactobacillus Starter Culture. Microorganisms 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosallaie, F.; Jooyandeh, H.; Hojjati, M.; Fazlara, A. Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci. Biotechnol. 2020, 29, 793–803. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why Are Weissella spp. Not Used as Commercial Starter Cultures for Food Fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Traisaeng, S.; Batsukh, A.; Chuang, T.-H.; Herr, D.R.; Huang, Y.-F.; Chimeddorj, B.; Huang, C.-M. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci. Rep. 2020, 10, 7928. [Google Scholar] [CrossRef]
- Tarrah, A.; Noal, V.; Giaretta, S.; Treu, L.; da Silva Duarte, V.; Corich, V.; Giacomini, A. Effect of different initial pH on the growth of Streptococcus macedonicus and Streptococcus thermophilus strains. Int. Dairy J. 2018, 86, 65–68. [Google Scholar] [CrossRef]
- Lee, A.H.; Neilson, A.P.; O’Keefe, S.F.; Ogejo, J.A.; Huang, H.; Ponder, M.; Chu, H.S.S.; Jin, Q.; Pilot, G.; Stewart, A.C. A laboratory-scale model cocoa fermentation using dried, unfermented beans and artificial pulp can simulate the microbial and chemical changes of on-farm cocoa fermentation. Eur. Food Res. Technol. 2019, 245, 511–519. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, C.; Guo, Y.; Wang, X.; Meng, Y. Polyphenols in fermented apple juice: Beneficial effects on human health. J. Funct. Foods 2021, 76, 104294. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Maciel, L.F.; de Souza Madureira Felício, A.L.; Miranda, L.C.R.; Pires, T.C.; da Silva Bispo, E.; Hirooka, E.Y. Aflatoxins and ochratoxin A in different cocoa clones (Theobroma cacao L.) developed in the southern region of Bahia, Brazil. Food Addit. Contam. Part A 2018, 35, 134–143. [Google Scholar] [CrossRef]
- dos Santos, D.G.; Coelho, C.C.; Ferreira, A.B.; Freitas-Silva, O. Brazilian Coffee Production and the Future Microbiome and Mycotoxin Profile Considering the Climate Change Scenario. Microorganisms 2021, 9, 858. [Google Scholar] [CrossRef]
- Ortiz, J.; Van Camp, J.; Mestdagh, F.; Donoso, S.; De Meulenaer, B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam. Part A 2013, 30, 2165–2176. [Google Scholar] [CrossRef]
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef]
- Ducos, C.; Pinson-Gadais, L.; Chereau, S.; Richard-Forget, F.; Vásquez-Ocmín, P.; Cerapio, J.P.; Casavilca-Zambrano, S.; Ruiz, E.; Pineau, P.; Bertani, S.; et al. Natural Occurrence of Mycotoxin-Producing Fusaria in Market-Bought Peruvian Cereals: A Food Safety Threat for Andean Populations. Toxins 2021, 13, 172. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B.; Shivakumara, P.; Shiva Kumar, J.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef]
- Guerrini, S.; Barbato, D.; Guerrini, L.; Mari, E.; Buscioni, G.; Mangani, S.; Romboli, Y.; Galli, V.; Parenti, A.; Granchi, L. Selection of Indigenous Saccharomyces cerevisiae Strains and Exploitation of a Pilot-Plant to Produce Fresh Yeast Starter Cultures in a Winery. Fermentation 2021, 7, 99. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Litwinek, D.; Boreczek, J.; Gambuś, H.; Buksa, K.; Berski, W.; Kowalczyk, M. Developing lactic acid bacteria starter cultures for wholemeal rye flour bread with improved functionality, nutritional value, taste, appearance and safety. PLoS ONE 2022, 17, e0261677. [Google Scholar] [CrossRef] [PubMed]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Lin, J.; Liu, Y. Biosensors Coupled with Signal Amplification Technology for the Detection of Pathogenic Bacteria: A Review. Biosensors 2021, 11, 190. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, L.S.; Cevallos-Cevallos, J.M.; Weckx, S.; Ruales, J. Traditional Fermented Foods from Ecuador: A Review with a Focus on Microbial Diversity. Foods 2022, 11, 1854. https://doi.org/10.3390/foods11131854
Guerra LS, Cevallos-Cevallos JM, Weckx S, Ruales J. Traditional Fermented Foods from Ecuador: A Review with a Focus on Microbial Diversity. Foods. 2022; 11(13):1854. https://doi.org/10.3390/foods11131854
Chicago/Turabian StyleGuerra, Luis Santiago, Juan Manuel Cevallos-Cevallos, Stefan Weckx, and Jenny Ruales. 2022. "Traditional Fermented Foods from Ecuador: A Review with a Focus on Microbial Diversity" Foods 11, no. 13: 1854. https://doi.org/10.3390/foods11131854