Fluidized-Bed Granulation of Probiotics-Encapsulated Spray-Dried Skim Milk Powder: Effects of a Fluidizing Aid, Moisture-Activation and Dehydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LGG-Fermented RSM
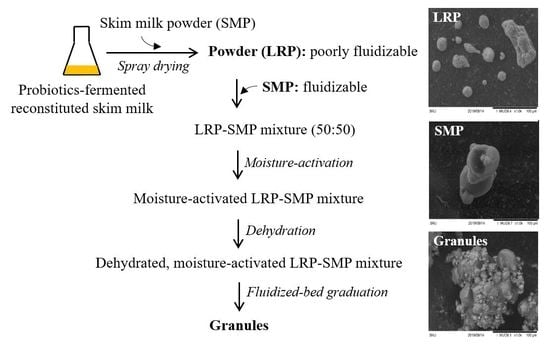
2.3. Spray Drying of LGG-Fermented RSM
2.4. Fluidized-Bed Granulation of Spray-Dried Powders
2.5. Microstructure and Particle Size Analyses
2.6. Density Measurement
2.7. Moisture Content and Water Activity Measurements
2.8. Determination of Flowability
2.9. Dispersibility Measurement
2.10. Survivability Measurement
2.11. Statistical Analysis
3. Results and Discussion
3.1. Geldart Classification of Spray-Dried Probiotic Powder
3.2. Effect of SMP on Fluidized-Bed Granulation of LRP
3.3. Effect of Moisture-Activation (without Dehydration) on Fluidized-Bed Granulation of LRP-SMP Mixture
3.4. Effect of Dehydration on the Properties of Moisture-Activated LRP-SMP Mixture
3.5. Effect of Moisture-Activation (with Dehydration) on Fluidized-Bed Granulation of LRP-SMP Mixture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martini, L.G.; Avontuur, P.; George, A.; Willson, R.J.; Crowley, P.J. Solubility parameter and oral absorption. Eur. J. Pharm. Biopharm. 1999, 48, 259–263. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005. [Google Scholar]
- Parikh, D.M. Handbook of Pharmaceutical Granulation Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Shanmugam, S. Granulation techniques and technologies: Recent progresses. BioImpacts 2015, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennis, B.J. Design and Optimization of Granulation Processes for Enhanced Product Performance; E&G Associates: Nashville, TN, USA, 1990. [Google Scholar]
- Narang, A.S.; Badawy, S.I. Handbook of Pharmaceutical Wet Granulation: Theory and Practice in a Quality by Design Paradigm; Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Thejaswini, P.; Suguna, B.; Sumalatha, N. Advanced granulation techniques for pharamceutical pharmulations. Int. J. Res. Pharm. Nano Sci. 2013, 2, 723–732. [Google Scholar]
- Khot, V.; Bhagwat, D.; D’Souza, J.; Shelake, S.; Patil, S. Optimization of granulation techniques for development of tablet dosage form. Indo Am. J. Pharm. Sci. 2017, 4, 4626. [Google Scholar]
- Friedman, M.; Donbrow, M.J. Fluidized bed coating technique for production of sustained release granules. Drug Dev. Ind. Pharm. 1978, 4, 319–331. [Google Scholar] [CrossRef]
- Schaafsma, S.; Vonk, P.; Kossen, N. Fluid bed agglomeration with a narrow droplet size distribution. Int. J. Pharm. 2000, 193, 175–187. [Google Scholar] [CrossRef]
- Cocco, R.; Karri, S.R.; Knowlton, T. Introduction to fluidization. Chem. Eng. Prog. 2014, 110, 21–29. [Google Scholar]
- Geldart, D. Types of gas fluidization. Powder Technol. 1973, 7, 285–292. [Google Scholar] [CrossRef]
- Kono, H.; Huang, C.; Morimoto, E.; Nakayama, T.; Hikosaka, T. Segregation and agglomeration of Type C powders from homogeneously aerated Type A–C powder mixtures during fluidization. Powder Technol. 1987, 53, 163–168. [Google Scholar] [CrossRef]
- Liu, Y.D.; Kimura, S. Fluidization and entrainment of difficult-to-fluidize fine powder mixed with easy-to-fluidize large particles. Powder Technol. 1993, 75, 189–196. [Google Scholar] [CrossRef]
- Ullah, I.; Wang, J.; Chang, S.Y.; Guo, H.; Kiang, S.; Jain, N. Moisture-activated dry granulation part II: The effects of formulation ingredients and manufacturing-process variables on granulation quality attributes. Pharm. Technol. 2009, 33, 42–51. [Google Scholar]
- Ullah, I.; Wang, J.; Chang, S.Y.; Wiley, G.J.; Jain, N.B.; Kiang, S. Moisture-activated dry granulation part I: A guide to excipient and equipment selection and formulation development. Pharm. Technol. 2009, 33, 62–70. [Google Scholar]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists (AOAC): Gaithersburg, MD, USA, 2005. [Google Scholar]
- United States Pharmacopeial Convention. USP 35-NF 30; United States Pharmacopeia: Rockville, MD, USA, 2012. [Google Scholar]
- Balde, A.; Aïdre, M. Effect of cryoconcentration, reverse osmosis and vacuum evaporation as concentration step of skim milk prior to drying on the powder properties. Powder Technol. 2017, 319, 463–471. [Google Scholar] [CrossRef]
- Schuck, P.; Dolivet, A.; Jeantet, R. Analytical Methods for Food and Dairy Powders; Wiley-Blackwell: West Sussex, UK, 2012. [Google Scholar]
- Broeckx, G.; Kiekens, S.; Jokicevic, K.; Byl, E.; Henkens, T.; Vandenheuvel, D.; Lebeer, S.; Kiekens, F. Effects of initial cell concentration, growth phase, and process parameters on the viability of Lactobacillus rhamnosus GG after spray drying. Dry. Technol. 2020, 38, 1474–1492. [Google Scholar] [CrossRef]
- Barkouti, A.; Turchiuli, C.; Carcel Carrión, J.A.; Dumoulin, E. Milk powder agglomerate growth and properties in fluidized bed agglomeration. Dairy Sci. Technol. 2013, 93, 523–535. [Google Scholar] [CrossRef]
- Palzer, S. Aggloeration of pharmaceutical, detergent, chemical and food powders-similarities and differences of materials and processes. Powder Technol. 2011, 206, 2–17. [Google Scholar] [CrossRef]
- Choi, J.H.; Suh, J.M.; Chang, I.Y.; Shun, D.W.; Yi, C.K.; Son, J.E.; Kim, S.D. The effect of fine particles on elutriation of coarse particles in a gas fluidized bed. Powder Technol. 2001, 121, 190–194. [Google Scholar] [CrossRef]
- Ma, X.; Kato, K. Effect of interparticle adhesion forces on elutriation of fine powders from a fluidized bed of a binary particle mixture. Powder Technol. 1998, 95, 93–101. [Google Scholar] [CrossRef]
- Tanaka, I.; Shinohara, H. Elutriation of fines from fluidized bed. J. Chem. Eng. Jpn. 1972, 5, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Turchiuli, C.; Smail, R.; Dumoulin, E. Fluidized bed agglomeration of skim milk powder: Analysis of sampling for the follow-up of agglomerate growth. Powder Technol. 2013, 238, 161–168. [Google Scholar] [CrossRef]
- Leroch, S.; Wendland, M. Influence of capillary bridge formation onto the silica nanoparticle interaction studied by grand canonical Monte Carlo simulations. Langmuir 2013, 29, 12410–12420. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Sakurai, A.; Katayama, T.; Matsuura, Y.; Ohyagi, N.; Wada, K.; Ishikawa, A.; Yonemochi, E. Novel, lean and environment-friendly granulation method: Green fluidized bed granulation (GFBG). Int. J. Pharm. 2019, 557, 18–25. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Laroche, C.; Fine, F.; Gervais, P. Water activity affects heat resistance of microorganisms in food powders. Int. J. Food Microbiol. 2005, 97, 307–315. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Gottardi, D.; Montanari, C.; Gianotti, A. Dynamic stresses of lactic acid bacteria associated to fermentation processes. In Lactic Acid Bacteria: R & D for Food, Health and Livestock Purposes; Kongo, J.M., Ed.; IntechOpen: London, UK, 2013; pp. 539–570. [Google Scholar]
- Tomas, J.; Kleinschmidt, S. Improvement of flowability of fine cohesive powders by flow additives. Chem. Eng. Technol. 2009, 32, 1470–1483. [Google Scholar] [CrossRef]
- Forny, L.; Marabi, A.; Palzer, S. Wetting, disintegration and dissolution of agglomerated water soluble powders. Powder Technol. 2011, 206, 72–78. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.P.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef] [PubMed]






| Particles 2 | ρlb (kg m−3) | ρtb (kg m−3) | CI | HR | Dispersibility (%) |
|---|---|---|---|---|---|
| LRP | 430 ± 0 b | 750 ± 0 a | 42.9 ± 0.01 a | 1.75 ± 0.00 a | 49.7 ± 5.9 c |
| LRP-G3 | 330 ± 0 c | 460 ± 20 c | 27.4 ± 2.28 b | 1.38 ± 0.04 b | 91.6 ± 2.0 b |
| SMP | 500 ± 10 a | 640 ± 10 b | 21.2 ± 0.64 c | 1.27 ± 0.01 c | 95.6 ± 1.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.-H.; Letona, A.; Lee, M.; Lim, D.; Han, N.-S.; Chung, D. Fluidized-Bed Granulation of Probiotics-Encapsulated Spray-Dried Skim Milk Powder: Effects of a Fluidizing Aid, Moisture-Activation and Dehydration. Foods 2021, 10, 1600. https://doi.org/10.3390/foods10071600
Lim D-H, Letona A, Lee M, Lim D, Han N-S, Chung D. Fluidized-Bed Granulation of Probiotics-Encapsulated Spray-Dried Skim Milk Powder: Effects of a Fluidizing Aid, Moisture-Activation and Dehydration. Foods. 2021; 10(7):1600. https://doi.org/10.3390/foods10071600
Chicago/Turabian StyleLim, Dong-Hyun, Andres Letona, Minjeong Lee, Dayoung Lim, Nam-Soo Han, and Donghwa Chung. 2021. "Fluidized-Bed Granulation of Probiotics-Encapsulated Spray-Dried Skim Milk Powder: Effects of a Fluidizing Aid, Moisture-Activation and Dehydration" Foods 10, no. 7: 1600. https://doi.org/10.3390/foods10071600