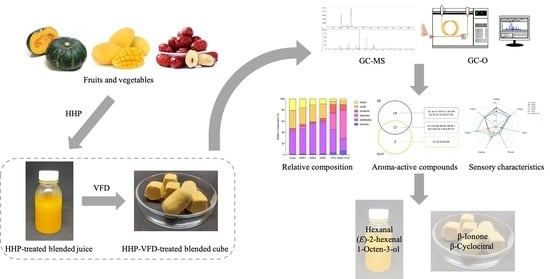
Effects of High Hydrostatic Pressure Combined with Vacuum-Freeze Drying on the Aroma-Active Compounds in Blended Pumpkin, Mango, and Jujube Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Juices Preparation
2.3. HHP of the Blended Juices
2.4. VFD of the Blended Cubes
2.5. Determination of Color Parameters
2.6. Microstructure Analysis
2.7. Analysis of Volatile Compounds
2.7.1. Isolation
2.7.2. GC–MS
2.7.3. Identification and Quantification
2.8. Gas Chromatography–Olfactometry (GC–O)
2.9. Odor Activity Value (OAV)
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of HHP and VFD on Color of Blended Juices
3.2. Effect of HHP on Microstructure
3.3. Effect of HHP and VFD on the Volatile Components of Blended Juices
3.3.1. Ketones
3.3.2. Aldehydes
3.3.3. Terpenes
3.3.4. Alcohols
3.3.5. Acids
3.3.6. Esters
3.4. Effect of Different Analytical Methods on the Detection of Aroma-Active Compounds
3.5. Flavor Profile
3.6. Comparative Sensory Analysis of Blended Juices with Different Processing Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lomeli-Martin, A.; Maria Martinez, L.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Induced changes in aroma compounds of foods treated with high hydrostatic pressure: A review. Foods 2021, 10, 878. [Google Scholar] [CrossRef]
- De Marchi, F.; Aprea, E.; Endrizzi, I.; Charles, M.; Betta, E.; Corollaro, M.L.; Cappelletti, M.; Ferrentino, G.; Spilimbergo, S.; Gasperi, F. Effects of pasteurization on volatile compounds and sensory properties of coconut (Cocos nucifera L.) water: Thermal vs. high-pressure carbon dioxide pasteurization. Food Bioprocess Technol. 2015, 8, 1393–1404. [Google Scholar] [CrossRef]
- Luo, D.; Pang, X.; Xu, X.; Bi, S.; Zhang, W.; Wu, J. Identification of cooked off-flavor components and analysis of their formation mechanisms in melon juice during thermal processing. J. Agric. Food Chem. 2018, 66, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Podolak, R.; Whitman, D.; Black, D.G. Factors affecting microbial inactivation during high pressure processing in juices and beverages: A review. J. Food Prot. 2020, 83, 1561–1575. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, P.; Lao, F.; Liu, J.; Liao, X.; Wu, J. Characterization of the major aroma-active compounds in Keitt mango juice: Comparison among fresh, pasteurization and high hydrostatic pressure processing juices. Food Chem. 2019, 289, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Kebede, B.T.; Doan Ngoc Hai, D.; Buve, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT Food Sci. Technol. 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, L.; Wang, Y.; Liao, X. Potential of high-pressure processing and high-temperature/short-time thermal processing on microbial, physicochemical and sensory assurance of clear cucumber juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 51–58. [Google Scholar] [CrossRef]
- Baxter, I.A.; Easton, K.; Schneebeli, K.; Whitfield, F.B. High pressure processing of Australian navel orange juices: Sensory analysis and volatile flavor profiling. Innov. Food Sci. Emerg. Technol. 2005, 6, 372–387. [Google Scholar] [CrossRef]
- Gomes, W.F.; Tiwari, B.K.; Rodriguez, O.; de Brito, E.S.; Narciso Fernandes, F.A.; Rodrigues, S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, X.; Han, W.; Wu, X.; Wang, Y.; Hu, X.; Ling, J.; Liao, X. Novel application of CO2-assisted high pressure processing in cucumber juice and apple juice. LWT Food Sci. Technol. 2018, 96, 491–498. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Hu, X.; Sun, Z.; Liao, X. Korla pear juice treated by ultrafiltration followed by high pressure processing or high temperature short time. LWT Food Sci. Technol. 2016, 65, 283–289. [Google Scholar] [CrossRef]
- Shishehgarha, F.; Makhlouf, J.; Ratti, C. Freeze-drying characteristics of strawberries. Dry. Technol. 2002, 20, 131–145. [Google Scholar] [CrossRef]
- Ul Hasan, M.; Malik, A.U.; Ali, S.; Imtiaz, A.; Munir, A.; Amjad, W.; Anwar, R. Modern drying techniques in fruits and vegetables to overcome postharvest losses: A review. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Ansar; Nazaruddin; Azis, A.D. New frozen product development from strawberries (Fragaria Ananassa Duch.). Heliyon 2020, 6, e05118. [Google Scholar] [CrossRef] [PubMed]
- Wojdylo, A.; Lech, K.; Nowicka, P.; Hernandez, F.; Figiel, A.; Antonio Carbonell-Barrachina, A. Influence of different drying techniques on phenolic compounds, antioxidant capacity and colour of Ziziphus jujube Mill. fruits. Molecules 2019, 24, 2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perfilova, O.V.; Akishin, D.V.; Vinnitskaya, V.F.; Danilin, S.I.; Olikainen, O.V. Use of vegetable and fruit powder in the production technology of functional food snacks. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 082071. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Colina-Coca, C.; Char, C.D.; Pilar Cano, M.; de Ancos, B.; Sanchez-Moreno, C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef]
- Colina-Coca, C.; de Ancos, B.; Sanchez-Moreno, C. Nutritional composition of processed onion: S-alk(en)yl-L-cysteine sulfoxides, organic acids, sugars, minerals, and vitamin C. Food Bioprocess Technol. 2014, 7, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, L.; Pan, X.; Lao, F.; Liao, X.; Wu, J. Alterations of phenolic compounds in red raspberry juice induced by high-hydrostatic-pressure and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2021, 67, 102569. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, Y.; Wang, C.; Liao, L.; Shi, D.; An, K.; Hu, J.; Wang, J.; Shi, L. Influence of high hydrostatic pressure pretreatment on properties of vacuum-freeze dried strawberry slices. Food Chem. 2020, 331, 127203. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, W.T.; Lao, F.; Mi, R.F.; Liao, X.J.; Luo, D.S.; Wu, J.H. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Xu, X.X.; Luo, D.S.; Lao, F.; Pang, X.L.; Shen, Q.; Hu, X.S.; Wu, J.H. Characterization of key aroma compounds in raw and roasted peas (Pisum sativum L.) by application of instrumental and sensory techniques. J. Agric. Food Chem. 2020, 68, 2718–2727. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.; Oliver, S.; Woosley, A.; Singleton, R.C. Sensory evaluation by quantitative description analysis. Food Technol. 1974, 28, 24–34. [Google Scholar]
- Stone, H.; Sidel, J.L.; Taylor, S. Sensory evaluation practices. In Food Science and Technology, 2nd ed.; Academic Press: Orlando, FL, USA, 1993; pp. 206–240. [Google Scholar]
- Koutchma, T.; Popovic, V.; Ros-Polski, V.; Popielarz, A. Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 844–867. [Google Scholar] [CrossRef] [Green Version]
- Izli, N.; Izli, G.; Taskin, O. Influence of different drying techniques on drying parameters of mango. Food Sci. Technol. 2017, 37, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, Z.; Pavkov, I.; Radojcin, M.; Horecki, A.T.; Keselj, K.; Kovacevic, D.B.; Putnik, P. Convective drying of fresh and frozen raspberries and change of their physical and nutritive properties. Foods 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Izli, N.; Polat, A. Freeze and convective drying of quince (Cydonia oblonga Miller.): Effects on drying kinetics and quality attributes. Heat Mass Transfer 2019, 55, 1317–1326. [Google Scholar] [CrossRef]
- Zvitov-Ya’ari, R.; Nussinovitch, A. Browning prevention in rehydrated freeze-dried non-blanched potato slices by electrical treatment. LWT Food Sci. Technol. 2014, 56, 194–199. [Google Scholar] [CrossRef]
- Dash, K.K.; Balasubramaniam, V.M.; Kamat, S. High pressure assisted osmotic dehydrated ginger slices. J. Food Eng. 2019, 247, 19–29. [Google Scholar] [CrossRef]
- Swami Hulle, N.R.; Rao, P.S. Effect of High pressure pretreatments on structural and dehydration characteristics of aloe vera (Aloe barbadensis Miller) cubes. Dry. Technol. 2016, 34, 105–118. [Google Scholar] [CrossRef]
- Pu, Y.; Ding, T.; Lv, R.; Cheng, H.; Liu, D. Effect of drying and storage on the volatile compounds of jujube fruit detected by electronic nose and GC-MS. Food Sci. Technol. Res. 2018, 24, 1039–1047. [Google Scholar] [CrossRef]
- Reche, J.; Hernandez, F.; Almansa, M.S.; Carbonell-Barrachina, A.A.; Legua, P.; Amoros, A. Effects of organic and conventional farming on the physicochemical and functional properties of jujube fruit. LWT Food Sci. Technol. 2019, 99, 438–444. [Google Scholar] [CrossRef]
- Kreck, M.; Patz, C.D.; Ludwig, M.; Degenhardt, A.; Paschold, P.; Dietrich, H. Influence of variety on carotinoid content and flavour in pumpkin juice. Dtsch. Lebensm.-Rundsch. 2004, 100, 445–452. [Google Scholar]
- Li, Y. Solid phase microextraction followed by GC-MS analysis of volatile flavor compounds in fresh pumpkin and pumpkin juice. Food Sci. 2010, 31, 208–210. [Google Scholar]
- Pino, J.A.; Mesa, J.; Munoz, Y.; Marti, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.L.; Pandey, S. Juice blends-a way of utilization of under-utilized fruits, vegetables, and spices: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.K.; King, E.S.; Ebeler, S.E.; Heymann, H. Characterizing the chemical and sensory profiles of United States Cabernet Sauvignon wines and blends. Am. J. Enol. Vitic. 2013, 64, 169–179. [Google Scholar] [CrossRef]
- Bi, S.; Sun, S.; Lao, F.; Liao, X.; Wu, J. Gas chromatography-mass spectrometry combined with multivariate data analysis as a tool for differentiating between processed orange juice samples on the basis of their volatile markers. Food Chem. 2020, 311, 125913. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, W.; Ma, L.; Xu, F.; Jin, P.; Zheng, Y. Effect of high pressure processing and thermal treatment on physicochemical parameters, antioxidant activity and volatile compounds of green asparagus juice. LWT Food Sci. Technol. 2015, 62, 927–933. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. High pressure treatments accelerate changes in volatile composition of sulphur dioxide-free wine during bottle storage. Food Chem. 2015, 188, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Krokida, M.K.; Philippopoulos, C. Volatility of apples during air and freeze drying. J. Food Eng. 2006, 73, 135–141. [Google Scholar] [CrossRef]
- Chin, S.T.; Nazimah, S.A.H.; Quek, S.Y.; Man, Y.B.C.; Rahman, R.A.; Hashim, D.M. Changes of volatiles’ attribute in durian pulp during freeze- and spray-drying process. LWT Food Sci. Technol. 2008, 41, 1899–1905. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K. Introduction to aroma compounds in foods. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–30. [Google Scholar]
- Hnin, K.K.; Zhang, M.; Wang, B. Impact of different FD-related drying methods on selected quality attributes and volatile compounds of rose flavored yogurt melts. Dry. Technol. 2021, 39, 1205–1218. [Google Scholar] [CrossRef]
- Musso, L.; Scaglia, B.; Al Haj, G.; Arnold, N.A.; Adani, F.; Scari, G.; Dallavalle, S.; Iriti, M. Chemical characterization and nematicidal activity of the essential oil of Nepeta nuda L. ssp pubescens and Nepeta curviflora Boiss. from lebanon. J. Essent. Oil Bear. Plants 2017, 20, 1424–1433. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lipids. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 158–247. [Google Scholar]
- Chen, Y.; Feng, X.; Ren, H.; Yang, H.; Liu, Y.; Gao, Z.; Long, F. Changes in physicochemical properties and volatiles of kiwifruit pulp beverage treated with high hydrostatic pressure. Foods 2020, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Anthon, G.E.; Barrett, D.M. Thermal inactivation of lipoxygenase and hydroperoxytrienoic acid lyase in tomatoes. Food Chem. 2003, 81, 275–279. [Google Scholar] [CrossRef]
- Rodrigo, D.; Jolie, R.; Loey, A.V.; Hendrickx, M. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase. J. Food Eng. 2007, 79, 423–429. [Google Scholar] [CrossRef]
- Wang, F.; Du, B.-L.; Cui, Z.-W.; Xu, L.-P.; Li, C.-Y. Effects of high hydrostatic pressure and thermal processing on bioactive compounds, antioxidant activity, and volatile profile of mulberry juice. Food Sci. Technol. Int. 2017, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Simancas, R.; Diaz-Maroto, M.C.; Perez-Coello, M.S.; Alanon, M.E. Viability of pre-treatment drying methods on mango peel by-products to preserve flavouring active compounds for its revalorisation. J. Food Eng. 2020, 279. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Menesatti, P.; Caparrotta, S.; Bazihizina, N.; Azzarello, E.; Petrucci, W.A.; Masi, E.; Giordani, E. Use of volatile organic compounds and physicochemical parameters for monitoring the post-harvest ripening of imported tropical fruits. Eur. Food Res. Technol. 2015, 241, 91–102. [Google Scholar] [CrossRef]
- Cheng, C.-x.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of Mandarin (Citrus unshiu) juice. Innov. Food Sci. Emerg. Technol. 2020, 64. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; Galvao, M.D.; Sandes, R.D.D.; Neta, M.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. LWT Food Sci. Technol. 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; Galvâo, M.d.S.; Leite Neta, M.T.S.; Dutra Sandes, R.D.; Mujumdar, A.S.; Narain, N. Comparative evaluation of physical properties and aroma profile of carrot slices subjected to hot air and freeze drying. Dry. Technol. 2017, 35, 699–708. [Google Scholar] [CrossRef]
- Diaz-Maroto, M.C.; Perez-Coello, M.S.; Cabezudo, M.D. Effect of drying method on the volatiles in bay leaf (Laurus nobilis L.). J. Agric. Food Chem. 2002, 50, 4520–4524. [Google Scholar] [CrossRef]
- de Torres, C.; Diaz-Maroto, M.C.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.; Naim, M. Citrus flavor stability. In Flavor Chemistry; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Verret, C.; Ballestra, P.; Cruz, C.; Pardon, P.; Largeteau, A.; El Moueffak, A.H. The effect of high hydrostatic pressure on black truffle (Tuber melanosporum) flavour compounds. J. Phys. Conf. Ser. 2008, 121, 142011. [Google Scholar] [CrossRef]
- Mirondo, R.; Barringer, S. Effect of peels on quality attributes of mango puree held for different times. J. Food Process. Preserv. 2017, 41, e12946. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Ripening temperatures influence biosynthesis of aroma volatile compounds in ‘Kensington Pride’ mango fruit. J. Hortic. Sci. Biotechnol. 2004, 79, 146–157. [Google Scholar] [CrossRef]
- Zabetakis, I.; Koulentianos, A.; Orruno, E.; Boyes, I. The effect of high hydrostatic pressure on strawberry flavour compounds. Food Chem. 2000, 71, 51–55. [Google Scholar] [CrossRef]
- Araya, X.I.T.; Smale, N.; Zabaras, D.; Winley, E.; Forde, C.; Stewart, C.M.; Mawson, A.J. Sensory perception and quality attributes of high pressure processed carrots in comparison to raw, sous-vide and cooked carrots. Innov. Food Sci. Emerg. Technol. 2009, 10, 420–433. [Google Scholar] [CrossRef]
- Wang, R.; Ding, S.; Zhao, D.; Wang, Z.; Wu, J.; Hu, X. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci. Biotechnol. 2016, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Bi, J.F.; Chen, Q.Q.; Wu, X.Y.; Lyu, Y.; Meng, X.J. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Gonzalez-Cebrino, F.; Garcia-Parra, J.; Ramirez, R. Aroma profile of a red plum puree processed by high hydrostatic pressure and analysed by SPME-GC/MS. Innov. Food Sci. Emerg. Technol. 2016, 33, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple aroma: Alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Bhandari, B.; Wang, B. Effects of infrared freeze drying on volatile profile, FTIR molecular structure profile and nutritional properties of edible rose flower (Rosa rugosaflower). J. Sci. Food Agric. 2020, 100, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.B.; Ramanathan, A.; Chennubhotla, C.S. Categorical dimensions of human odor descriptor space revealed by non-negative matrix factorization. PLoS ONE 2013, 8, e0073289. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic characterization of mangoes (Mangifera indica L.) using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.C.; Xiao, Z.B. Characterization of the major odor-active compounds in dry jujube cultivars by application of gas chromatography-olfactometry and odor activity value. J. Agric. Food Chem. 2018, 66, 7722–7734. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, J.; Wang, Y.; Wang, X.; Chen, F.; Wang, X. Characterization of aroma-impact compounds in dry jujubes (Ziziphus jujube Mill.) by aroma extract dilution analysis (AEDA) and gas chromatography-mass spectrometer (GC-MS). Int. J. Food Prop. 2018, 21, 1844–1853. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Parra, J.; Gonzalez-Cebrino, F.; Ramirez, R. Volatile compounds of a pumpkin (Cucurbita moschata) puree processed by high pressure thermal processing. J. Sci. Food Agric. 2020, 100, 4449–4456. [Google Scholar] [CrossRef]
- Pan, X.; Wu, J.; Zhang, W.; Liu, J.; Yang, X.; Liao, X.; Hu, X.; Lao, F. Effects of sugar matrices on the release of key aroma compounds in fresh and high hydrostatic pressure processed Tainong mango juices. Food Chem. 2021, 338, 128117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.-C.; Molnar, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechoff, A.; Dhuique-Mayer, C.; Dornier, M.; Tomlins, K.I.; Boulanger, R.; Dufour, D.; Westby, A. Relationship between the kinetics of β-carotene degradation and formation of norisoprenoids in the storage of dried sweet potato chips. Food Chem. 2010, 121, 348–357. [Google Scholar] [CrossRef]
- Jeyaprakash, S.; Heffernan, J.E.; Driscoll, R.H.; Frank, D.C. Impact of Dry. Technologies on tomato flavor composition and sensory quality. LWT Food Sci. Technol. 2020, 120, 108888. [Google Scholar] [CrossRef]
- Tandon, K.S.; Baldwin, E.A.; Scott, J.W.; Shewfelt, R.L. Linking sensory descriptors to volatile and nonvolatile components of fresh tomato flavor. J. Food Sci. 2003, 68, 2366–2371. [Google Scholar] [CrossRef]
- Xing, Y.; Lei, H.; Wang, J.; Wang, Y.; Wang, J.; Xu, H. Effects of different drying methods on the total phenolic, rosmarinic acid and essential oil of purple perilla leaves. J. Essent. Oil Bear. Plants 2017, 20, 1594–1606. [Google Scholar] [CrossRef]
- Xu, J.; He, Z.; Zeng, M.; Li, B.; Qin, F.; Wang, L.; Wu, S.; Chen, J. Effect of xanthan gum on the release of strawberry flavor in formulated soy beverage. Food Chem. 2017, 228, 595–601. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, D.; Zhang, W.; Lorenzo, J.M. Recent advantage of interactions of protein-flavor in foods: Perspective of theoretical models, protein properties and extrinsic factors. Trends Food Sci. Technol. 2021, 111, 405–425. [Google Scholar] [CrossRef]
- Rajkumar, G.; Rajan, M.; Araujo, H.C.; Jesus, M.S.; Leite Neta, M.T.S.; Sandes, R.D.D.; Narain, N. Comparative evaluation of volatile profile of tomato subjected to hot air, freeze, and spray drying. Dry. Technol. 2020, 39, 383–391. [Google Scholar] [CrossRef]
- Luo, W.; Tappi, S.; Wang, C.F.; Yu, Y.; Zhu, S.M.; Rocculi, P. Study and optimization of high hydrostatic pressure (HHP) to improve mass transfer and quality characteristics of candied green plums (Prunus mume). J. Food Process. Preserv. 2018, 42, 13769. [Google Scholar] [CrossRef]






| L* | a* | b* | C* | h0 | ΔE | ||
|---|---|---|---|---|---|---|---|
| Juices | 0 MPa (Fresh) | 36.58 ± 0.25 c | 6.05 ± 0.03b c | 17.45 ± 0.29 bc | 18.47 ± 0.27 bc | 70.88 ± 0.35 bc | - |
| 200 MPa (HHP1) | 36.81 ± 0.03 c | 6.20 ± 0.26 b | 17.26 ± 0.13 c | 18.34 ± 0.21 c | 70.24 ± 0.63 c | 0.46 ± 0.12 c | |
| 400 MPa (HHP2) | 36.86 ± 0.14 c | 6.15 ± 0.02b c | 17.69 ± 0.43 b | 18.73 ± 0.40 bc | 70.81 ± 0.48 bc | 0.45 ± 0.43 c | |
| 600 MPa (HHP3) | 36.70 ± 0.06 c | 5.96 ± 0.09 c | 17.81 ± 0.01 b | 18.78 ± 0.03 b | 71.50 ± 0.26 b | 0.48 ± 0.26 c | |
| VFD-treated rehydrated juices | 40.98 ± 0.02 b | 6.68 ± 0.11 a | 21.52 ± 0.05 a | 22.54 ± 0.04 a | 72.77 ± 0.29 a | 5.62 ± 0.03 b | |
| HHP3–VFD-treated rehydrated juices | 41.25 ± 0.04 a | 6.85 ± 0.07 a | 21.78 ± 0.10 a | 22.83 ± 0.11 a | 72.53 ± 0.12 a | 6.01 ± 0.09 a | |
| Cubes | 0 MPa (VFD) | 62.36 ± 0.17 | 17.52 ± 0.08 | 52.82 ± 0.14 | 55.65 ± 0.16 | 71.65 ± 0.04 | - |
| 600 MPa (HHP3–VFD) | 63.77 ± 0.68 | 17.94 ± 0.37 | 53.00 ± 0.33 | 55.95 ± 0.43 | 71.30 ± 0.25 | 1.49 ± 0.62 |
| No. | Compounds | CAS | LRI 1 | Concentration (μg/kg) 2 | Identification 3 | Origin 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HHP-Treated | HHP–VFD-Treated | ||||||||||
| 0 MPa (Fresh) | 200 MPa (HHP1) | 400 MPa (HHP2) | 600 MPa (HHP3) | 0 MPa (VFD) | 600 MPa (HHP3–VFD) | ||||||
| Ketones | |||||||||||
| A1 | Acetone | 67-64-1 | 755 | 4.09 ± 0.73 a | 2.40 ± 0.05 b | 1.97 ± 0.11 c | 1.89 ± 0.23 c | 1.23 ± 0.12 d | n.d. | MS, LRI | Mango |
| A2 | 2-Butanone | 78-93-3 | 888 | n.d. | 2.06 ± 0.30 a | n.d. | n.d. | n.d. | n.d. | MS, LRI | Pumpkin |
| A3 | Ethyl vinyl ketone | 1629-58-9 | 1010 | n.d. | n.d. | n.d. | 2.26 ± 0.63 a | n.d. | n.d. | MS, LRI | Jujube, mango |
| A4 | 1-(1,3-Dimethyl-3-cyclohexen-1-yl) ethanone | 51733-68-7 | 1137 | 4.33 ± 0.15 a | 3.44 ± 0.32 b | 2.54 ± 0.13 c | 1.79 ± 0.32 d | n.d. | n.d. | MS | Other |
| A5 | Acetoin | 513-86-0 | 1274 | 5.57 ± 0.96 b | 1.91 ± 0.67 c | 1.86 ± 0.23 c | 2.23 ± 0.34 c | n.d. | 18.60 ± 2.03 a | MS, LRI | Pumpkin |
| A6 | 6-Methyl-5-hepten-2-one | 110-93-0 | 1325 | 1.01 ± 0.08 d | 1.13 ± 0.21 c | 2.45 ± 0.10 b | 1.23 ± 0.11 c | 4.15 ± 0.14 a | 2.52 ± 0.03 b | MS, LRI | Jujube, pumpkin, mango |
| A7 | 4’-Methyl acetophenone | 122-00-9 | 1751 | n.d. | n.d. | n.d. | 1.28 ± 0.08 a | n.d. | n.d. | MS, LRI | Pumpkin |
| A8 | β-Damascenone | 23726-93-4 | 1771 | n.d. | n.d. | 1.50 ± 0.33 c | 1.62 ± 0.11 b | n.d. | 3.14 ± 0.42 a | MS, LRI | Pumpkin |
| A9 | Geranylacetone | 3796-70-1 | 1822 | n.d. | n.d. | n.d. | n.d. | 4.26 ± 0.44 b | 6.18 ± 1.66 a | MS, LRI | Pumpkin, mango |
| A10 | β-Ionone | 79-77-6 | 1896 | n.d. | n.d. | n.d. | n.d. | 9.33 ± 0.73 b | 10.65 ± 1.91 a | MS, LRI | Jujube, pumpkin |
| Subtotal | 15.00 ± 1.92 c | 10.94 ± 1.55 d | 10.32 ± 0.90 d | 12.30 ± 1.82 d | 18.97 ± 1.43 b | 41.09 ± 6.05 a | |||||
| Aldehydes | |||||||||||
| B1 | Acetaldehyde | 75-07-0 | 682 | 7.11 ± 1.04 a | 2.17 ± 0.80 b | 2.35 ± 0.40 b | 2.19 ± 0.67 b | 1.77 ± 0.26 b | n.d. | MS, LRI | Mango |
| B2 | 2-Methylbutyraldehyde | 96-17-3 | 866 | n.d. | n.d. | n.d. | n.d. | 2.42 ± 1.83 a | n.d. | MS, LRI | Pumpkin |
| B3 | Isovaleraldehyde | 590-86-3 | 878 | 4.10 ± 0.39 ab | 1.69 ± 0.38 b | 2.22 ± 0.35 b | 1.76 ± 0.26 b | 4.71 ± 0.71 a | 5.18 ± 4.51 a | MS, LRI | Mango |
| B4 | Valeraldehyde | 110-62-3 | 928 | 15.59 ± 3.80 ab | 9.35 ± 1.06 c | 19.41 ± 1.95 a | 15.85 ± 4.46 ab | 11.84 ± 1.17 bc | 4.29 ± 1.06 d | MS, LRI | Pumpkin |
| B5 | Hexanal | 66-25-1 | 1070 | 22.26 ± 2.43 c | 17.74 ± 1.38 c | 77.88 ± 4.70 b | 102.59 ± 7.16 a | 23.12 ± 1.69 c | 8.81 ± 0.90 d | MS, LRI | Jujube, pumpkin, mango |
| B6 | 3-Methyl-2-butenal | 107-86-8 | 1199 | n.d. | n.d. | n.d. | n.d. | 4.78 ± 0.63 a | 3.56 ± 0.30 b | MS, LRI | Pumpkin |
| B7 | Heptaldehyde | 111-71-7 | 1174 | n.d. | n.d. | n.d. | 2.96 ± 0.23 a | n.d. | 1.76 ± 0.14 b | MS, LRI | Pumpkin |
| B8 | (E)-2-Hexenal | 6728-26-3 | 1207 | 393.53 ± 96.22 a | 146.01 ± 27.53 b | 145.29 ± 24.56 b | 148.52 ± 26.43 b | 86.48 ± 4.38 c | 56.47 ± 35.73 d | MS, LRI | Jujube, pumpkin, mango |
| B9 | Furfural | 98-01-1 | 1439 | 8.24 ± 1.19 a | 2.75 ± 0.78 b | 3.27 ± 0.61 b | 2.77 ± 0.59 b | 2.72 ± 0.59 b | 3.12 ± 0.15 b | MS, LRI | Jujube, pumpkin |
| B10 | (E)-2-Heptenal | 18829-55-5 | 1309 | 5.24 ± 0.61 c | 1.36 ± 0.06 d | 10.24 ± 0.54 ab | 8.61 ± 0.93 b | 11.32 ± 0.07 a | 6.00 ± 3.48 c | MS, LRI | Jujube, mango |
| B11 | 1-Nonanal | 124-19-6 | 1377 | n.d. | n.d. | 1.89 ± 0.28 b | 3.30 ± 0.48 a | 1.96 ± 0.30 b | n.d. | MS, LRI | Jujube, pumpkin, mango |
| B12 | 5-Ethyl-1-cyclopentene-1-carboxaldehyde | 36431-60-4 | 1393 | n.d. | n.d. | 3.29 ± 0.13 b | 3.52 ± 0.27 a | n.d. | n.d. | MS | Other |
| B13 | (E)-2-Octenal | 2548-87-0 | 1408 | n.d. | n.d. | 8.18 ± 0.38 b | 7.49 ± 0.65 c | 8.95 ± 0.19 a | 7.08 ± 0.92 c | MS, LRI | Jujube |
| B14 | (E, E)-2,4-Heptadienal | 4313-03-5 | 1446 | n.d. | n.d. | n.d. | n.d. | 4.07 ± 0.60 a | 4.01 ± 0.62 a | MS, LRI | Jujube, pumpkin |
| B15 | Benzaldehyde | 100-52-7 | 1499 | 58.31 ± 5.12 a | 29.67 ± 2.51 c | 39.17 ± 6.10 b | 32.94 ± 3.97 bc | 12.24 ± 0.08 d | 11.80 ± 2.68 d | MS, LRI | Jujube, pumpkin |
| B16 | (E)-2-Nonenal | 18829-56-6 | 1511 | 17.67 ± 16.53 a | 2.70 ± 1.08 b | 3.73 ± 1.15 b | 3.80 ± 0.39 b | 7.37 ± 1.06 ab | n.d. | MS, LRI | Jujube, mango |
| B17 | (E,Z)-2,6-Nonadienal | 557-48-2 | 1561 | 21.53 ± 3.78 a | n.d. | n.d. | 1.84 ± 0.16 d | 8.43 ± 0.12 b | 5.58 ± 3.43 c | MS, LRI | Mango |
| B18 | β-Cyclocitral | 432-25-7 | 1589 | n.d. | n.d. | n.d. | n.d. | 7.80 ± 0.23 a | 7.17 ± 0.57 b | MS, LRI | Pumpkin |
| B19 | 5-Hydroxymethylfurfural | 67-47-0 | 2499 | n.d. | n.d. | n.d. | n.d. | 1.69 ± 0.30 a | n.d. | MS, LRI | Mango |
| Subtotal | 553.58 ± 131.11 a | 213.44 ± 35.58 c | 316.92 ± 41.15 b | 338.14 ± 46.65 b | 201.67 ± 14.21 c | 124.83 ± 54.46 d | |||||
| Terpenes | |||||||||||
| C1 | 3-Carene | 13466-78-9 | 1126 | 10.23 ± 2.44 c | 9.26 ± 2.31 c | 10.10 ± 1.06 c | 8.64 ± 1.87 c | 101.94 ± 1.09 b | 162.76 ± 19.51 a | MS, LRI | Mango |
| C2 | α-Phellandrene | 99-83-2 | 1133 | n.d. | n.d. | n.d. | n.d. | 2.18 ± 0.12 b | 3.79 ± 0.07 a | MS, LRI | Jujube, mango |
| C3 | Myrcene | 123-35-3 | 1150 | n.d. | n.d. | n.d. | n.d. | 2.48 ± 0.16 b | 4.01 ± 1.28 a | MS, LRI | Jujube, mango |
| C4 | β-Thujene | 28634-89-1 | 1146 | n.d. | n.d. | n.d. | n.d. | 2.40 ± 0.19 b | 3.97 ± 0.40 a | MS, LRI | Mango |
| C5 | 4-Carene | 29050-33-7 | 1166 | 15.24 ± 4.86 ab | 11.15 ± 1.94 bc | 6.95 ± 1.83 cd | 2.43 ± 0.26 d | 19.18 ± 4.51 a | 3.35 ± 0.55 d | MS, LRI | Mango |
| C6 | DL-Limonene | 138-86-3 | 1175 | n.d. | n.d. | n.d. | n.d. | 6.55 ± 1.23 b | 14.95 ± 2.93 a | MS, LRI | Jujube, mango |
| C7 | Terpinolene | 586-62-9 | 1266 | 15.28 ± 3.78 b | 11.11 ± 0.65 c | n.d. | n.d. | n.d. | 92.78 ± 5.20 a | MS, LRI | Jujube, mango |
| C8 | α, p-Dimethylstyrene | 1195-32-0 | 1416 | n.d. | n.d. | n.d. | n.d. | 6.95 ± 0.28 a | 8.51 ± 5.15 a | MS, LRI | Mango |
| Subtotal | 40.75 ± 11.08 c | 31.52 ± 4.90 c | 17.05 ± 2.89 d | 11.07 ± 2.13 d | 141.68 ± 7.58 b | 294.12 ± 35.08 a | |||||
| Alcohols | |||||||||||
| D1 | 1-Penten-3-ol | 616-25-1 | 1158 | n.d. | n.d. | n.d. | n.d. | 2.33 ± 0.10 a | n.d. | MS, LRI | Jujube, pumpkin |
| D2 | 2-Methyl-1-butanol | 137-32-6 | 1202 | n.d. | n.d. | n.d. | n.d. | n.d. | 10.81 ± 0.98 a | MS, LRI | Jujube, mango |
| D3 | 1-Pentanol | 71-41-0 | 1244 | 6.18 ± 0.19 ab | 2.50 ± 0.91 c | 5.64 ± 0.64 b | 8.10 ± 2.50 a | n.d. | n.d. | MS, LRI | Jujube, pumpkin, mango |
| D4 | (E)-3-Hexen-1-ol | 928-97-2 | 1370 | 13.54 ± 0.87 a | 5.92 ± 1.49 c | 6.43 ± 0.84 c | 9.76 ± 2.12 b | 2.02 ± 0.08 d | n.d. | MS, LRI | Jujube, mango |
| D5 | 1-Octen-3-ol | 3391-86-4 | 1435 | 5.15 ± 1.81 d | 7.43 ± 0.32 c | 8.33 ± 2.38 c | 10.89 ± 1.95 b | 17.07 ± 0.75 a | 10.43 ± 0.92 b | MS, LRI | Jujube, pumpkin, mango |
| D6 | p-Cymen-8-ol | 1197-01-9 | 1798 | n.d. | n.d. | n.d. | n.d. | 9.79 ± 0.97 a | 10.08 ± 1.98 a | MS, LRI | Mango |
| D7 | Benzyl alcohol | 100-51-6 | 1820 | n.d. | n.d. | n.d. | n.d. | 1.26 ± 0.22 b | 2.37 ± 1.01 a | MS, LRI | Jujube |
| D8 | Carveol | 99-48-9 | 1857 | n.d. | n.d. | n.d. | n.d. | 43.31 ± 1.05 a | 26.11 ± 6.56 b | MS, LRI | Mango |
| Subtotal | 24.87 ± 2.87 d | 15.85 ± 2.72 e | 20.40 ± 3.86 e | 28.75 ± 6.57 c | 75.78 ± 3.17 a | 59.80 ± 11.45 b | |||||
| Acids | |||||||||||
| E1 | Acetic acid | 64-19-7 | 1435 | 191.64 ± 32.42 a | 59.12 ± 25.37 b | 64.19 ± 4.98 b | 63.49 ± 16.09 b | 4.84 ± 0.90 c | 8.38 ± 2.40 c | MS, LRI | Jujube, pumpkin |
| E2 | Propionic acid | 79-09-4 | 1522 | 5.02 ± 0.63 a | 2.15 ± 0.16 b | 2.05 ± 0.38 b | 1.69 ± 0.28 b | n.d. | n.d. | MS, LRI | Jujube |
| E3 | Butanoic acid | 107-92-6 | 1608 | 9.44 ± 2.00 a | 2.49 ± 0.79 bc | 2.90 ± 0.70 b | 2.46 ± 0.64 bc | 1.03 ± 0.15 c | 3.24 ± 0.77 b | MS, LRI | Pumpkin, mango |
| E4 | 2-Methyl butanoic acid | 116-53-0 | 1647 | 17.30 ± 3.74 a | 5.63 ± 1.85 b | 6.88 ± 1.41 b | 5.58 ± 1.19 b | n.d. | n.d. | MS, LRI | Pumpkin, mango |
| E5 | Valeric acid | 109-52-4 | 1707 | 12.05 ± 1.47 a | 3.67 ± 0.94 b | 4.96 ± 1.18 b | 4.23 ± 0.77 b | n.d. | n.d. | MS, LRI | Pumpkin |
| E6 | Hexanoic acid | 142-62-1 | 1797 | 115.90 ± 17.58 a | 40.13 ± 10.51 b | 60.32 ± 15.41 b | 47.12 ± 8.68 b | 8.57 ± 1.36 c | 12.75 ± 2.81 c | MS, LRI | Jujube, pumpkin, mango |
| E7 | Heptanoic acid | 111-14-8 | 1884 | 37.33 ± 3.83 a | 13.58 ± 3.51 b | 17.89 ± 4.80 b | 14.81 ± 2.06 b | 6.21 ± 1.44 c | 6.39 ± 2.41 c | MS, LRI | Jujube |
| E8 | Octanoic acid | 124-07-2 | 2009 | 19.34 ± 0.92 a | 7.81 ± 1.78 bc | 9.69 ± 2.60 b | 8.44 ± 1.25 bc | 7.05 ± 2.19 bc | 5.81 ± 2.72 c | MS, LRI | Jujube, pumpkin, mango |
| E9 | Benzoic acid | 65-85-0 | 2391 | 16.57 ± 3.67 a | 4.30 ± 1.01 b | 4.96 ± 2.04 b | 5.33 ± 0.88 b | n.d. | n.d. | MS, LRI | Jujube, pumpkin |
| Subtotal | 424.59 ± 66.26 a | 138.88 ± 45.92 b | 173.84 ± 33.50 b | 153.15 ± 31.84 b | 27.70 ± 6.04 c | 36.57 ± 11.11 c | |||||
| Esters | |||||||||||
| F1 | Methyl acetate | 79-20-9 | 764 | 4.85 ± 1.18 a | 1.82 ± 0.47 b | 1.81 ± 0.22 b | 2.01 ± 0.21 b | 1.23 ± 0.01 b | n.d. | MS, LRI | Jujube, pumpkin |
| F2 | Ethyl acetate | 141-78-6 | 852 | 40.19 ± 14.98 a | 10.63 ± 8.20 b | 5.75 ± 1.10 b | 5.99 ± 3.12 b | 5.20 ± 1.67 b | 10.4 ± 5.68 b | MS, LRI | Jujube |
| F3 | Methyl butyrate | 623-42-7 | 986 | 72.25 ± 4.34 a | 19.45 ± 9.50 b | 12.61 ± 2.79 b | 11.73 ± 4.01 b | 1.62 ± 0.10 c | 2.82 ± 1.51 c | MS, LRI | Jujube, mango |
| F4 | Ethyl butyrate | 105-54-4 | 1028 | 97.67 ± 49.16 a | 14.30 ± 13.10 b | 5.49 ± 2.03 b | 3.94 ± 1.86 b | n.d. | n.d. | MS, LRI | Jujube, mango |
| F5 | Methyl valerate | 624-24-8 | 1076 | 9.54 ± 1.37 a | 4.08 ± 0.71 c | 5.77 ± 0.86 b | 5.25 ± 0.81 bc | n.d. | n.d. | MS, LRI | Jujube |
| F6 | Isoamyl acetate | 123-92-2 | 1113 | 4.72 ± 0.75 a | n.d. | n.d. | n.d. | n.d. | n.d. | MS, LRI | Mango |
| F7 | Methyl hexanoate | 106-70-7 | 1178 | 30.90 ± 3.93 a | 15.6 ± 1.94 c | 23.71 ± 3.11 b | 18.08 ± 2.41 c | 3.88 ± 0.30 d | n.d. | MS, LRI | Jujube, mango |
| F8 | Methyl heptanoate | 106-73-0 | 1278 | 3.79 ± 0.19 a | 2.25 ± 0.15 c | 3.43 ± 0.41 a | n.d. | 2.72 ± 0.46 b | 2.44 ± 0.17 bc | MS, LRI | Jujube |
| F9 | Methyl benzoate | 93-58-3 | 1595 | 9.77 ± 1.70 a | 5.39 ± 0.40 c | 7.71 ± 1.64 b | 5.98 ± 0.57 bc | 6.27 ± 0.04 bc | 5.25 ± 0.95 c | MS, LRI | Jujube |
| F10 | 3-Phenylpropionic acid methyl ester | 103-25-3 | 1854 | n.d. | 1.64 ± 0.13 b | 2.39 ± 0.49 a | 1.85 ± 0.25 b | n.d. | n.d. | MS, LRI | Jujube |
| Subtotal | 273.68 ± 77.60 a | 75.16 ± 34.60 b | 68.67 ± 12.65 b | 54.83 ± 13.24 c | 20.92 ± 2.58 d | 20.91 ± 8.31 d | |||||
| Total | 1332.47 ± 290.84 a | 485.79 ± 125.27 c | 607.2 ± 94.95 b | 598.24 ± 102.25 b | 486.72 ± 35.01 c | 577.32 ± 126.46 b | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Liang, X.; Pan, X.; Lao, F.; Shi, Y.; Wu, J. Effects of High Hydrostatic Pressure Combined with Vacuum-Freeze Drying on the Aroma-Active Compounds in Blended Pumpkin, Mango, and Jujube Juice. Foods 2021, 10, 3151. https://doi.org/10.3390/foods10123151
Yuan L, Liang X, Pan X, Lao F, Shi Y, Wu J. Effects of High Hydrostatic Pressure Combined with Vacuum-Freeze Drying on the Aroma-Active Compounds in Blended Pumpkin, Mango, and Jujube Juice. Foods. 2021; 10(12):3151. https://doi.org/10.3390/foods10123151
Chicago/Turabian StyleYuan, Lin, Xujuan Liang, Xin Pan, Fei Lao, Yong Shi, and Jihong Wu. 2021. "Effects of High Hydrostatic Pressure Combined with Vacuum-Freeze Drying on the Aroma-Active Compounds in Blended Pumpkin, Mango, and Jujube Juice" Foods 10, no. 12: 3151. https://doi.org/10.3390/foods10123151