Different Methods of Scan Alignment in Erosive Tooth Wear Measurements: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
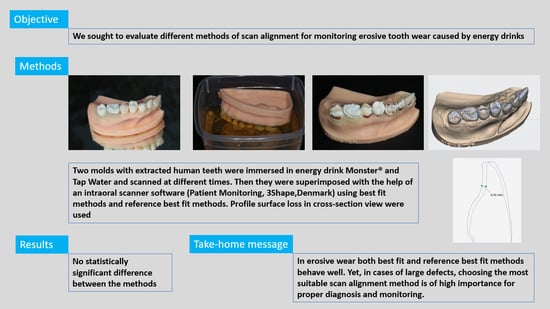
2.2. Erosive Solutions and 3D Scanning
2.3. Alignment Methods
2.4. Precision Assessment
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, K.; Wilmes, B.; Grandjean, C.; Drescher, D. Impact of manual control point selection accuracy on automated surface matching of digital dental models. Clin. Oral Investig. 2018, 22, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, J.L.; Kurland, B.F.; King, G.J.; Wheeler, T.T.; Ghafari, J.; Ramsay, D.S. A 3-dimensional analysis of molar movement during headgear treatment. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 18–29, discussion 29–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; He, L.; Guo, H.M.; Tian, J.; Bai, Y.X.; Li, S. Integrated three-dimensional digital assessment of accuracy of anterior tooth movement using clear aligners. Korean J. Orthod. 2015, 45, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Schlenz, M.A.; Schlenz, M.B.; Wöstmann, B.; Jungert, A.; Ganss, C. Intraoral scanner-based monitoring of tooth wear in young adults: 12-month results. Clin. Oral Investig. 2022, 26, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Rebele, S.F.; Zuhr, O.; Schneider, D.; Jung, R.E.; Hürzeler, M.B. Tunnel technique with connective tissue graft versus coronally advanced flap with enamel matrix derivative for root coverage: A RCT using 3D digital measuring methods. Part II. Volumetric studies on healing dynamics and gingival dimensions. J. Clin. Periodontol. 2014, 41, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, R.P.; Tveit, A.B.; Wiegand, A. Consensus report of the European Federation of Conservative Dentistry: Erosive tooth wear-diagnosis and management. Clin. Oral Investig. 2015, 19, 1557–1561. [Google Scholar] [CrossRef]
- Warreth, A.; Abuhijleh, E.; Almaghribi, M.A.; Mahwal, G.; Ashawish, A. Tooth surface loss: A review of literature. Saudi Dent. J. 2020, 32, 53–60. [Google Scholar] [CrossRef]
- Alsunni, A.A. Energy Drink Consumption: Beneficial and Adverse Health Effects. Int. J. Health Sci. 2015, 9, 468–474. [Google Scholar] [CrossRef]
- Ghozayel, M.; Ghaddar, A.; Farhat, G.; Nasreddine, L.; Kara, J.; Jomaa, L. Energy drinks consumption and perceptions among University Students in Beirut, Lebanon: A mixed methods approach. PLoS ONE 2020, 15, e0232199. [Google Scholar] [CrossRef]
- Alford, C.; Cox, H.; Wescott, R. The effects of red bull energy drink on human performance and mood. Amino Acids 2001, 21, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Jaeggi, T.; Zero, D. The role of diet in the aetiology of dental erosion. Caries Res. 2004, 38 (Suppl. S1), 34–44. [Google Scholar] [CrossRef]
- Joshi, M.; Joshi, N.; Kathariya, R.; Angadi, P.; Raikar, S. Techniques to Evaluate Dental Erosion: A Systematic Review of Literature. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE07. [Google Scholar] [CrossRef]
- Chadwick, R.; Mitchell, H.; Cameron, I.; Hunter, B.; Tulley, M. Development of a novel system for assessing tooth and restoration wear. J. Dent. 1997, 25, 41–47. [Google Scholar] [CrossRef]
- Dixon, B.; Sharif, M.O.; Ahmed, F.; Smith, A.B.; Seymour, D.; Brunton, P.A. Evaluation of the basic erosive wear examination (BEWE) for use in general dental practice. Br. Dent. J. 2012, 213, E4. [Google Scholar] [CrossRef] [PubMed]
- Tantbirojn, D.; Pintado, M.R.; Versluis, A.; Dunn, C.; Delong, R. Quantitative analysis of tooth surface loss associated with gastroesophageal reflux disease: A longitudinal clinical study. J. Am. Dent. Assoc. 2012, 143, 278–285. [Google Scholar] [CrossRef]
- Chadwick, R.G.; Mitchell, H.L.; Manton, S.L.; Ward, S.; Ogston, S.; Brown, R. Maxillary incisor palatal erosion: No correlation with dietary variables? J. Clin. Pediatr. Dent. 2005, 29, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Pintado, M.R.; Anderson, G.C.; DeLong, R.; Douglas, W.H. Variation in tooth wear in young adults over a two-year period. J. Prosthet. Dent. 1997, 77, 313–320. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Austin, R.S.; Bartlett, D.W. A method to evaluate profilometric tooth wear measurements. Dent. Mater. 2012, 28, 245–251. [Google Scholar] [CrossRef]
- Schlenz, M.A.; Schlenz, M.B.; Wöstmann, B.; Glatt, A.S.; Ganss, C. Intraoral scanner-based monitoring of tooth wear in young adults: 24-month results. Clin. Oral Investig. 2023, 27, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Witecy, C.; Ganss, C.; Wöstmann, B.; Schlenz, M.B.; Schlenz, M.A. Monitoring of Erosive Tooth Wear with Intraoral Scanners In vitro. Caries Res. 2021, 55, 215–224. [Google Scholar] [CrossRef]
- Besl, P.; McKay, H.D. A method for registration of 3-D shapes. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 239–256. [Google Scholar] [CrossRef]
- Park, S.Y.; Subbarao, M. A fast point-to-tangent plane technique for multi-view registration. In Proceedings of the Fourth International Conference on 3-D Digital Imaging and Modeling, Banff, AB, Canada, 6–10 October 2003. 3DIM 2003, Proceedings. [Google Scholar]
- Howard, M.A., 3rd; Dobbs, M.B.; Simonson, T.M.; LaVelle, W.E.; Granner, M.A. A noninvasive, reattachable skull fiducial marker system. Technical note. J. Neurosurg. 1995, 83, 372–376. [Google Scholar] [CrossRef]
- Wulfman, C.; Koenig, V.; Mainjot, A.K. Wear measurement of dental tissues and materials in clinical studies: A systematic review. Dent. Mater. 2018, 34, 825–850. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, S.; Osnes, C.; Bartlett, D.; Keeling, A. Investigation into the accuracy and measurement methods of sequential 3D dental scan alignment. Dent. Mater. 2019, 35, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Westendorff, C.; Leitner, C.; Bartz, D.; Reinert, S. Validation of 3D-laser surface registration for image-guided cranio-maxillofacial surgery. J. Craniomaxillofac. Surg. 2005, 33, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.A.; Mohamed, A.M.F.S.; Pau, A. Morphological patterns of the palatal rugae: A review. J. Oral Biosci. 2020, 62, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kratzsch, H.; Opitz, C. Investigations on the palatal rugae pattern in cleft patients. Part. II: Changes in the distances from the palatal rugae to maxillary points. J. Orofac. Orthop. 2000, 61, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wegehaupt, F.J.; Wiegand, A.; Roos, M.; Attin, T.; Buchalla, W. Erosion and abrasion of tooth-colored restorative materials and human enamel. J. Dent. 2009, 37, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, J.F.; Duggal, M.S.; Malik-Kotru, G.; Curzon, M.E. Soft drinks and dental health: A review of the current literature. J. Dent. 2006, 34, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Luka, B. Erosive tooth wear—A review on global prevalence and on its prevalence in risk groups. Br. Dent. J. 2018, 22, 364–370. [Google Scholar] [CrossRef]
- Jarvinen, V.K.; Rytomaa, I.I.; Heinonen, O.P. Risk factors in dental erosion. J. Dent. Res. 1991, 70, 942–947. [Google Scholar] [CrossRef]
- Dugmore, C.R.; Rock, W.P. A multifactorial analysis of factors associated with dental erosion. Br. Dent. J. 2004, 196, 283–286. [Google Scholar] [CrossRef]
- Al-Malik, M.I.; Holt, R.D.; Bedi, R. The relationship between erosion, caries and rampant caries and dietary habits in preschool children in Saudi Arabia. Int. J. Paediatr. Dent. 2001, 11, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.G.; Martins, J.P.; de Sousa, E.B.; Fernandes, N.L.; Meira, I.A.; Sampaio, F.C.; de Oliveira, A.F.; Pereira, A.M. Influence of energy drinks on enamel erosion: In vitro study using different assessment techniques. J. Clin. Exp. Dent. 2021, 13, e1076–e1082. [Google Scholar] [CrossRef]
- Bronkhorst, H.; Bronkhorst, E.; Kalaykova, S.; Pereira-Cenci, T.; Huysmans, M.C.; Loomans, B. Inter- and intra-variability in tooth wear progression at surface-, tooth- and patient-level over a period of three years: A cohort study.: Inter- and intra-variation in tooth wear progression. J. Dent. 2023, 138, 104693. [Google Scholar] [CrossRef] [PubMed]
- Michou, S.; Vannahme, C.; Ekstrand, K.R.; Benetti, A.R. Detecting early erosive tooth wear using an intraoral scanner system. J. Dent. 2020, 100, 103445. [Google Scholar] [CrossRef]
- Kouri, V.; Moldovani, D.; Papazoglou, E. Accuracy of Direct Composite Veneers via Injectable Resin Composite and Silicone Matrices in Comparison to Diagnostic Wax-Up. J. Funct. Biomater. 2023, 14, 32. [Google Scholar] [CrossRef]
- Lambrechts, P.; Braem, M.; Vuylsteke-Wauters, M.; Vanherle, G. Quantitative in vivo wear of human enamel. J. Dent. Res. 1989, 68, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Al-Omiri, M.K.; Harb, R.; Abu Hammad, O.A.; Lamey, P.J.; Lynch, E.; Clifford, T.J. Quantification of tooth wear: Conventional vs new method using toolmakers microscope and a three-dimensional measuring technique. J. Dent. 2010, 38, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Blunt, L.; Smith, B.G. Measurement of tooth wear in patients with palatal erosion. Br. Dent. J. 1997, 182, 179–184. [Google Scholar] [CrossRef]
- Schmalzl, J.; Róth, I.; Borbély, J.; Hermann, P.; Vecsei, B. The impact of software updates on accuracy of intraoral scanners. BMC Oral Health 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Gonzalez, B.; Setyo, C.; Picaza, M.G.; Tribst, J.P.M. Effect of defect size and tooth anatomy in the measurements of a 3D patient monitoring tool. Heliyon 2022, 8, e12103. [Google Scholar] [CrossRef] [PubMed]
- Mulic, A.; Ruud, A.; Stenhagen, I.R.; Bruzell, E.; Tulek, A. Deterioration of direct restorative materials under erosive conditions with impact of abrasion and attrition in vitro. Biomater. Investig. Dent. 2023, 10, 2202211. [Google Scholar] [CrossRef] [PubMed]
- Finer, Y.; Santerre, J.P. Biodegradation of a dental composite by esterases: Dependence on enzyme concentration and specificity. J. Biomater. Sci. Polym. Ed. 2003, 14, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Nica, I.; Iovan, G.; Ghiorghe, A.; Stoleriu, S.; Pancu, G.; Andrian, S. Chemical Corrosion of Nano-filled Composite Resins in Various Solutions. Mater. Plast. 2014, 3, 282–285. [Google Scholar]
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of flowable resin composite surfaces eroded by acidic and alcoholic drinks. Dent. Mater. J. 2008, 27, 455–465. [Google Scholar] [CrossRef]
- Lee, Y.K.; Powers, J.M. Discoloration of dental resin composites after immersion in a series of organic and chemical solutions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 361–367. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Chew, C.L.; Ong, L.F.K.L.; Teoh, S.H. Environmental damage and occlusal contact area wear of composite restoratives. J. Oral Rehabil. 2002, 29, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Stoleriu, S.; Andrian, S.; Pancu, G.; Nica, I.; Munteanu, A.; Balan, A.; Iovan, G. The effect of erosion and abrasion on surface properties of composite resin. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012056. [Google Scholar] [CrossRef]
- Abu-Bakr, N.H.; Han, L.; Okamoto, A.; Iwaku, M. Effect of Alcoholic and Low-pH Soft Drinks on Fluoride Release from Compomer. J. Esthet. Restor. Dent. 2000, 12, 97–104. [Google Scholar] [CrossRef]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- O’Toole, S.; Osnes, C.; Bartlett, D.; Keeling, A. Investigation into the validity of WearCompare, a purpose-built software to quantify erosive tooth wear progression. Dent. Mater. 2019, 35, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, S.; Bartlett, D.; Keeling, A.; McBride, J.; Bernabe, E.; Crins, L.; Loomans, B. Influence of Scanner Precision and Analysis Software in Quantifying Three-Dimensional Intraoral Changes: Two-Factor Factorial Experimental Design. J. Med. Internet Res. 2020, 22, e17150. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Mancino, D.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderón, J.E.; Kafa, L.; Etienne, O.; et al. Treatment of Tooth Wear Using Direct or Indirect Restorations: A Systematic Review of Clinical Studies. Bioengineering 2022, 9, 346. [Google Scholar] [CrossRef]
- Bartlett, D.; O’Toole, S. Tooth Wear: Best Evidence Consensus Statement. J. Prosthodont. 2020, 30, 20–25. [Google Scholar] [CrossRef]





| Time | Monster® | Tap Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Best Fit | Tooth Comparison | Fillings | Palatal Rugae | Best Fit | Tooth Comparison | Fillings | Palatal Rugae | |
| 1 h | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 3 h | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 6 h | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) | 0.01 (0) a | 0.01 (0) a |
| 12 h | 0.01 (0) a | 0.01 (0) | 0.01 (0) | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 1 d | 0.01 (0) a | 0.01 (0) a | 0.01 (0.01) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 2 d | 0.05 (0.02) a | 0.02 (0.05) a | 0.02 (0.03) a | 0.01 (0.01) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 4 d | 0.13 (0.04) b | 0.08 (0.07) b | 0.1 (0.14) b | 0.08 (0.12) b | 0.01 (0) a | 0.01 (0.01) a | 0.01 (0) a | 0.01 (0) a |
| 1 w | 0.27 (0.07) b | 0.22 (0.07) b | 0.24 (0.08) b | 0.21 (0.1) b | 0.01 (0.05) a | 0.01 (0) a | 0.01 (0) a | 0.01 (0) a |
| 2 w | 0.62 (0.09) c | 0.45 (0.2) c | 0.67 (0.11) c | 0.59 (0.07) c | 0.01 (0.03) a | 0.02 (0.02) a | 0.01 (0) a | 0.01 (0) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loumprinis, N.; Michou, S.; Rahiotis, C. Different Methods of Scan Alignment in Erosive Tooth Wear Measurements: An In Vitro Study. Dent. J. 2024, 12, 34. https://doi.org/10.3390/dj12020034
Loumprinis N, Michou S, Rahiotis C. Different Methods of Scan Alignment in Erosive Tooth Wear Measurements: An In Vitro Study. Dentistry Journal. 2024; 12(2):34. https://doi.org/10.3390/dj12020034
Chicago/Turabian StyleLoumprinis, Nikolaos, Stavroula Michou, and Christos Rahiotis. 2024. "Different Methods of Scan Alignment in Erosive Tooth Wear Measurements: An In Vitro Study" Dentistry Journal 12, no. 2: 34. https://doi.org/10.3390/dj12020034