Pain Assessment in Oral Medicine through Its Different Dimensions: A Comprehensive Review
Abstract
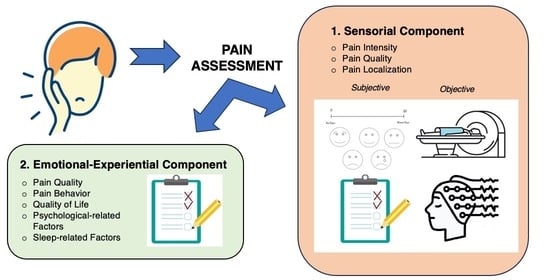
:1. Introduction
- Pain is a personal experience, shaped by biological, psychological, and social factors.
- Pain and nociception are distinct concepts, with the former being notably more intricate.
- People develop their understanding of pain throughout their lives.
- When a patient communicates pain, their expression deserves maximum respect.
- Despite its protective and adaptive roles, pain can negatively affect psychological and social well-being.
- Verbal description stands as just one method to portray pain [6].
2. Materials and Methods
2.1. Focused Questions
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Research
2.5. Quality Assessment of Included Studies
3. Results
Risk of Bias
4. Discussion
4.1. Pain Intensity Assessment
Pain Intensity Assessment in Children
4.2. Pain Localization Assessment
4.3. Pain Quality Assessment
- The Pain Rating Index (PRI), which refers to PI, globally and for each class.
- Constellation Words, which are the words commonly used to describe pain related to a specific condition. The adjectives belonging to this definition are usually the ones used by at least 33% of patients who have that condition. The most used words for describing pain in burning mouth syndrome (BMS) are, for example, “hot/burning”, “radiating”, “tiring/exhausting”, and “fearful” [57].
- The Number of Words Chosen (NWC), which corresponds to the total number of words chosen, with the frequency rate of each.
4.4. Pain Behavior Assessment
4.5. Quality of Life Assessment
4.6. Psychological Assessment
4.6.1. Anxiety and Depression Assessment
4.6.2. Pain Coping Mechanism Assessment
4.7. Sleep Disorder Assessment
4.8. Global Pain Assessment
4.9. Research Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menendez, M.E.; Ring, D. Factors Associated with Greater Pain Intensity. Hand Clin. 2015, 32, 27–31. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthr. Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Hajihasani, A.; Rouhani, M.; Salavati, M.; Hedayati, R.; Kahlaee, A.H. The Influence of Cognitive Behavioral Therapy on Pain, Quality of Life, and Depression in Patients Receiving Physical Therapy for Chronic Low Back Pain: A Systematic Review. PM&R 2019, 11, 167–176. [Google Scholar] [CrossRef]
- Kazi, A.M.; Khalid, W. Questionnaire designing and validation. J. Pak. Med. Assoc. 2012, 62, 514–516. [Google Scholar]
- Xu, X.; Huang, Y. Objective Pain Assessment: A Key for the Management of Chronic Pain. F1000Research 2020, 9, 35. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, N.M.; Chen, C.; Gilam, G.; Mackey, S.; Scherrer, G. Brain circuits for pain and its treatment. Sci. Transl. Med. 2021, 13, eabj7360. [Google Scholar] [CrossRef]
- Gorczyca, R.; Filip, R.; Walczak, E. Psychological aspects of pain. Ann. Agric. Environ. Med. 2013, S1, 23–27. [Google Scholar]
- Pigg, M.; Law, A.; Nixdorf, D.; Renton, T.; Sharav, Y.; Svensson, P.; Ernberg, M.; Peck, C.; Alstergren, P.; Kaspo, G.; et al. International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [Google Scholar] [CrossRef]
- Lin, Y.; De Araujo, I.; Stanley, G.; Small, D.; Geha, P. Chronic pain precedes disrupted eating behavior in low-back pain patients. PLoS ONE 2022, 17, e0263527. [Google Scholar] [CrossRef]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef]
- Rogers, A.H.; Farris, S.G. A meta-analysis of the associations of elements of the fear-avoidance model of chronic pain with negative affect, depression, anxiety, pain-related disability and pain intensity. Eur. J. Pain 2022, 26, 1611–1635. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The Association of Sleep and Pain: An Update and a Path Forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Whibley, D.; AlKandari, N.; Kristensen, K.; Barnish, M.; Rzewuska, M.; Druce, K.L.; Tang, N.K. Sleep and Pain: A Systematic Review of Studies of Mediation. Clin. J. Pain 2019, 35, 544–558. [Google Scholar] [CrossRef]
- Treister, R.; Honigman, L.; Lawal, O.D.; Lanier, R.K.; Katz, N.P. A deeper look at pain variability and its relationship with the placebo response: Results from a randomized, double-blind, placebo-controlled clinical trial of naproxen in osteoarthritis of the knee. Pain 2019, 160, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Shafshak, T.S.; Elnemr, R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J. Clin. Rheumatol. 2021, 27, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, D.B.; Strout, T.D. The minimum clinically significant difference in patient-assigned numeric scores for pain. Am. J. Emerg. Med. 2005, 23, 828–832. [Google Scholar] [CrossRef]
- Taddio, A.; O’brien, L.; Ipp, M.; Stephens, D.; Goldbach, M.; Koren, G. Reliability and validity of observer ratings of pain using the visual analog scale (VAS) in infants undergoing immunization injections. Pain 2009, 147, 141–146. [Google Scholar] [CrossRef]
- Todd, K.H.; Funk, K.G.; Funk, J.P.; Bonacci, R. Clinical significance of reported changes in pain severity. Ann. Emerg. Med. 1996, 27, 485–489. [Google Scholar] [CrossRef]
- Closs, S.; Barr, B.; Briggs, M.; Cash, K.; Seers, K. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J. Pain Symptom Manag. 2004, 27, 196–205. [Google Scholar] [CrossRef]
- Lewinson, R.T.; Wiley, J.P.; Worobets, J.T.; Stefanyshyn, D.J. Development and validation of a computerized visual analog scale for the measurement of pain in patients with patellofemoral pain syndrome. Clin. J. Sport Med. 2013, 23, 392–396. [Google Scholar] [CrossRef]
- Ruskin, D.; Lalloo, C.; Amaria, K.; Stinson, J.N.; Kewley, E.; Campbell, F.; Brown, S.C.; Jeavons, M.; A McGrath, P. Assessing pain intensity in children with chronic pain: Convergent and discriminant validity of the 0 to 10 numerical rating scale in clinical practice. Pain Res. Manag. 2014, 19, 141–148. [Google Scholar] [CrossRef]
- Wikström, L.; Nilsson, M.; Broström, A.; Eriksson, K. Patients’ self-reported nausea: Validation of the Numerical Rating Scale and of a daily summary of repeated Numerical Rating Scale scores. J. Clin. Nurs. 2019, 28, 959–968. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test–retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Jenkins, H.H.; Spencer, E.D.; Weissgerber, A.J.; Osborne, L.A.; Pellegrini, J.E. Correlating an 11-point verbal numeric rating scale to a 4-point verbal rating scale in the measurement of pruritus. J. Perianesth Nurs. 2009, 24, 152–155. [Google Scholar] [CrossRef]
- Hicks, C.L.; von Baeyer, C.L.; Spafford, P.A.; van Korlaar, I.; Goodenough, B. The Faces Pain Scale–Revised: Toward a common metric in pediatric pain measurement. Pain 2001, 93, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Suraseranivongse, S.; Montapaneewat, T.; Manon, J.; Chainchop, P.; Petcharatana, S.; Kraiprasit, K. Cross-validation of a self-report scale for postoperative pain in school-aged children. J. Med. Assoc. Thai. 2005, 88, 412–418. [Google Scholar] [PubMed]
- Sun, T.; West, N.; Ansermino, J.M.; Montgomery, C.J.; Myers, D.; Dunsmuir, D.; Lauder, G.R.; von Baeyer, C.L. A smartphone version of the Faces Pain Scale-Revised and the Color Analog Scale for postoperative pain assessment in children. Pediatr. Anesthesia 2015, 25, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Cravero, J.P.; Fanciullo, G.J.; McHugo, G.J.; Baird, J.C. The validity of the Computer Face Scale for measuring pediatric pain and mood. Pediatr. Anesthesia 2013, 23, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Fadayevatan, R.; Alizadeh-Khoei, M.; Hessami-Azar, S.T.; Sharifi, F.; Haghi, M.; Kaboudi, B. Validity and Reliability of 11-face Faces Pain Scale in the Iranian Elderly Community with Chronic Pain. Indian J. Palliat. Care 2019, 25, 46–51. [Google Scholar]
- Lee, J.J.; Lee, M.K.; Kim, J.E.; Kim, H.Z.; Park, S.H.; Tae, J.H.; Choi, S.S. Pain relief scale is more highly correlated with numerical rating scale than with visual analogue scale in chronic pain patients. Pain Physician 2015, 18, E195-200. [Google Scholar] [PubMed]
- Giraudeau, B.; Rozenberg, S.; Valat, J.-P. Assessment of the clinically relevant change in pain for patients with sciatica. Ann. Rheum. Dis. 2004, 63, 1180–1181. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Castarlenas, E.; de la Vega, R.; Solé, E.; Tomé-Pires, C.; Jensen, M.; Engel, J.; Racine, M. Validity of three rating scales for measuring pain intensity in youths with physical disabilities. Eur. J. Pain 2016, 20, 130–137. [Google Scholar] [CrossRef]
- Malara, A.; De Biase, G.A.; Bettarini, F.; Ceravolo, F.; Di Cello, S.; Garo, M.; Praino, F.; Settembrini, V.; Sgrò, G.; Spadea, F.; et al. Pain Assessment in Elderly with Behavioral and Psychological Symptoms of Dementia. J. Alzheimer’s Dis. 2016, 50, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Ersek, M.; Herr, K.; Neradilek, M.B.; Buck, H.G.; Black, B. Comparing the Psychometric Properties of the Checklist of Nonverbal Pain Behaviors (CNPI) and the Pain Assessment in Advanced Dementia (PAIN-AD) Instruments. Pain Med. 2010, 11, 395–404. [Google Scholar] [CrossRef]
- Paulson-Conger, M.; Leske, J.; Maidl, C.; Hanson, A.; Dziadulewicz, L. Comparison of two pain assessment tools in nonverbal critical care patients. Pain Manag. Nurs. 2011, 12, 218–224. [Google Scholar] [CrossRef]
- De-Figueiredo, F.E.D.; Lima, L.F.; Lima, G.S.; Oliveira, L.S.; Ribeiro, M.A.; Brito-Junior, M.; Correa, M.B.; Sousa-Neto, M.; e Silva, A.L.F. Apical periodontitis healing and postoperative pain following endodontic treatment with a reciprocating single-file, single-cone approach: A randomized controlled pragmatic clinical trial. PLoS ONE 2020, 15, e0227347. [Google Scholar] [CrossRef]
- Tran, H.T.; Kong, Y.; Talati, A.; Posada-Quintero, H.; Chon, K.H.; Chen, I. The use of electrodermal activity in pulpal diagnosis and dental pain assessment. Int. Endod. J. 2023, 56, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Odai, E.D.; Ehizele, A.O.; Enabulele, J.E. Assessment of pain among a group of Nigerian dental patients. BMC Res. Notes 2015, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Mahajan, A.; Shah, N.; Dadhania, A.P. Preemptive analgesia in third molar impaction surgery. Natl. J. Maxillofac. Surg. 2012, 3, 144–147. [Google Scholar] [CrossRef]
- Khatri, A.; Kalra, N. A comparison of two pain scales in the assessment of dental pain in East delhi children. ISRN Dent. 2012, 2012, 247351. [Google Scholar] [CrossRef]
- Versloot, J.; Veerkamp, J.S.J.; Hoogstraten, J. Dental Discomfort Questionnaire: Predicting toothache in preverbal children. Eur. J. Paediatr. Dent. 2004, 5, 170–173. [Google Scholar]
- Felipak, P.K.; Menoncin, B.L.V.; Reyes, M.R.T.; Costa, L.R.; Souza, J.F.; Menezes, J.V.N.B. Determinants of parental report of dental pain and discomfort in preschool children—The Dental Discomfort Questionnaire. Int. J. Paediatr. Dent. 2020, 30, 436–444. [Google Scholar] [CrossRef]
- Daher, A.; Abreu, M.H.; Costa, L.R. Recognizing preschool children with primary teeth needing dental treatment because of caries-related toothache. Community Dent. Oral Epidemiol. 2015, 43, 298–307. [Google Scholar] [CrossRef]
- Senirkentli, G.B.; Tirali, R.E.; Bani, M. Assessment of dental pain in children with intellectual disability using the dental discomfort questionnaire. J. Intellect. Disabil. 2021, 26, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.R.; Noll, M.; Silveira, E.A. Adaptation and validation of body maps for musculoskeletal pain location in patients with severe obesity. Korean J. Pain 2018, 31, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Aibel, K.; Moldwin, R. Validation of the ‘Pelvic Pain Map’: A new self-assessment tool for chronic pelvic pain localisation. BJU Int. 2023, 131, 763–769. [Google Scholar] [CrossRef]
- Elson, D.; Jones, S.; Caplan, N.; Stewart, S.; Gibson, A.S.C.; Kader, D. The photographic knee pain map: Locating knee pain with an instrument developed for diagnostic, communication and research purposes. Knee 2011, 18, 417–423. [Google Scholar] [CrossRef]
- Adamo, D.; Pecoraro, G.; Fortuna, G.; Amato, M.; Marenzi, G.; Aria, M.; Mignogna, M.D. Assessment of oral health-related quality of life, measured by OHIP-14 and GOHAI, and psychological profiling in burning mouth syndrome: A case-control clinical study. J. Oral Rehabil. 2020, 47, 42–52. [Google Scholar] [CrossRef]
- Sevrain, M.; Brenaut, E.; Le Toux, G.; Misery, L. Primary Burning Mouth Syndrome: A Questionnaire Study of Neuropathic and Psychological Components. Am. J. Clin. Dermatol. 2016, 17, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Katz, J.; Jeans, M.E. The role of compensation in chronic pain: Analysis using a new method of scoring the McGill Pain Questionnaire. Pain 1985, 23, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kachooei, A.R.; Ebrahimzadeh, M.H.; Erfani-Sayyar, R.; Salehi, M.; Salimi, E.; Razi, S. Short Form-McGill Pain Questionnaire-2 (SF-MPQ-2): A Cross-Cultural Adaptation and Validation Study of the Persian Version in Patients with Knee Osteoarthritis. Arch. Bone Jt. Surg. 2015, 3, 45–50. [Google Scholar] [PubMed]
- Fontana Carvalho, A.P.; Dufresne, S.S.; Rogerio de Oliveira, M.; Couto Furlanetto, K.; Dubois, M.; Dallaire, M.; Ngomo, S.; da Silva, R.A. Effects of lumbar stabilization and muscular stretching on pain, disabilities, postural control and muscle activation in pregnant woman with low back pain. Eur. J. Phys. Rehabil. Med. 2020, 56, 297–306. [Google Scholar] [CrossRef]
- França, F.R.; Burke, T.N.; Hanada, E.S.; Marques, A.P. Segmental stabilization and muscular strengthening in chronic low back pain-a comparative study. Clinics 2010, 65, 1013–1017. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Trudeau, J.J.; Benson, C.; Biondi, D.M.; Katz, N.P.; Kim, M. Validation of the Short-form McGill Pain Questionnaire-2 (SF-MPQ-2) in acute low back pain. J. Pain 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Erdogan, O.; Malek, M.; Janal, M.N.; Gibbs, J.L. Sensory testing associates with pain quality descriptors during acute dental pain. Eur. J. Pain 2019, 23, 1701–1711. [Google Scholar] [CrossRef]
- Lewandowski, A.S.M.; Palermo, T.M.; Kirchner, H.L.; Drotar, D. Comparing diary and retrospective reports of pain and activity restriction in children and adolescents with chronic pain conditions. Clin. J. Pain 2009, 25, 299–306. [Google Scholar] [CrossRef]
- Vertsberger, D.; Talmon, A.; Ziadni, M.; Kong, J.-T.; Darnall, B.D.; Manber, R.; Mackey, S.; Gross, J.J. Intensity of Chronic Low Back Pain and Activity Interference: A Daily Diary Study of the Moderating Role of Cognitive Pain Coping Strategies. Pain Med. 2023, 24, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Karoly, P.; Okun, M.A.; Enders, C.; Tennen, H. Effects of pain intensity on goal schemas and goal pursuit: A daily diary study. Health Psychol. 2014, 33, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, P.; Stammen, C.; Bissantz, N.; Jensen, M.P. Pain vs. comfort diary: A fully remote app-based experiment. Eur. J. Pain 2019, 23, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Jain, K.; Singh, J.; Saxena, P.; Nyima, T.; Selvam, S.R.; Walia, M.C. Clinical Utility of the Behavioral Pain Assessment Tool in Patients Admitted in the Intensive Care Unit. Indian J. Crit. Care Med. 2020, 24, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Ok, S.; Ho, D.; Lynd, T.; Cheon, K. Evaluation of children’s pain expression and behavior using audio visual distraction. Clin. Exp. Dent. Res. 2021, 7, 795–802. [Google Scholar] [CrossRef]
- Gomarverdi, S.; Sedighie, L.; Seifrabiei, M.A.; Nikooseresht, M. Comparison of Two Pain Scales: Behavioral Pain Scale and Critical-care Pain Observation Tool During Invasive and Noninvasive Procedures in Intensive Care Unit-admitted Patients. Iran. J. Nurs. Midwifery Res. 2019, 24, 151–155. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Verneuer, B.; Dany, K.; Marziniak, M.; Wolowski, A.; Çolak-Ekici, R.; Schulte, T.L.; Bullmann, V.; Grewe, S.; Gralow, I.; et al. Validation of the pain sensitivity questionnaire in chronic pain patients. Pain 2012, 153, 1210–1218. [Google Scholar] [CrossRef]
- Sellers, A.B.; Ruscheweyh, R.; Kelley, B.J.; Ness, T.J.; Vetter, T.R. Validation of the English language pain sensitivity questionnaire. Reg. Anesth. Pain Med. 2013, 38, 508–514. [Google Scholar] [CrossRef]
- Bell, B.A.; Ruscheweyh, R.; Kelley, B.J.; Ness, T.J.; Vetter, T.R.; Sellers, A.B. Ethnic Differences Identified by Pain Sensitivity Questionnaire Correlate With Clinical Pain Responses. Reg. Anesth. Pain Med. 2018, 43, 200–204. [Google Scholar] [CrossRef]
- Heary, K.O.; Wong, A.W.K.; Lau, S.C.L.; Dengler, J.; Thompson, M.R.; Crock, L.W.; Novak, C.B.; Philip, B.A.; Mackinnon, S.E. Quality of Life and Psychosocial Factors as Predictors of Pain Relief Following Nerve Surgery. Hand 2022, 17, 193–199. [Google Scholar] [CrossRef]
- Müller, R.; Landmann, G.; Béchir, M.; Hinrichs, T.; Arnet, U.; Jordan, X.; Brinkhof, M.W.G. Chronic pain, depression and quality of life in individuals with spinal cord injury: Mediating role of participation. J. Rehabil. Med. 2017, 49, 489–496. [Google Scholar] [CrossRef]
- Kwan, Y.H.; Fong, W.W.S.; Lui, N.L.; Yong, S.T.; Cheung, Y.B.; Malhotra, R.; Østbye, T.; Thumboo, J. Validity and reliability of the Short Form 36 Health Surveys (SF-36) among patients with spondyloarthritis in Singapore. Rheumatol. Int. 2016, 36, 1759–1765. [Google Scholar] [CrossRef]
- Kishi, M.; Abe, A.; Yonemitsu, M. Relationship between the SF-36 questionnaire and patient’s satisfaction following halitosis therapy. Oral Dis. 2005, 11 (Suppl. S1), 89–91. [Google Scholar] [CrossRef]
- Campos, L.A.; Peltomäki, T.; Marôco, J.; Campos, J.A.D.B. Use of Oral Health Impact Profile-14 (OHIP-14) in Different Contexts. What Is Being Measured? Int. J. Environ. Res. Public Health 2021, 18, 13412. [Google Scholar] [CrossRef]
- Omara, M.; Salzberger, T.; Boecker, M.; Bekes, K.; Steiner, G.; Nell-Duxneuner, V.; Ritschl, V.; Mosor, E.; Kloppenburg, M.; Sautner, J.; et al. Improving the measurement of oral health-related quality of life: Rasch model of the oral health impact profile-14. J. Dent. 2021, 114, 103819. [Google Scholar] [CrossRef]
- Musskopf, M.L.; Milanesi, F.C.; da Rocha, J.M.; Fiorini, T.; Moreira, C.H.C.; Susin, C.; Rösing, C.K.; Weidlich, P.; Oppermann, R.V. Oral health related quality of life among pregnant women: A randomized controlled trial. Braz. Oral Res. 2018, 32, e002. [Google Scholar] [CrossRef] [PubMed]
- Yule, P.L.; Durham, J.; Playford, H.; Moufti, M.A.; Steele, J.; Steen, N.; Wassell, R.W.; Ohrbach, R. OHIP-TMDs: A patient-reported outcome measure for temporomandibular disorders. Community Dent. Oral Epidemiol. 2015, 43, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; López-Pintor, R.M.; Fernández-Castro, M.; Ramírez, L.; Sanz, M.; López, J.; Blázquez, M.; González, J.J.; Hernández, G.; EPOX-SSp Group. Usefulness of implementing the OHIP -14 questionnaire to assess the impact of xerostomia and hyposalivation on quality of life in patients with primary Sjögren’s syndrome. J. Oral Pathol. Med. 2022, 51, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Chana, P.; Smith, J.G.; Karamat, A.; Simpson, A.; Renton, T. Catastrophising, pain self-efficacy and acceptance in patients with Burning Mouth Syndrome. J. Oral Rehabil. 2021, 48, 458–468. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Lucero-Berdugo, M. Quality of life in patients with burning mouth syndrome. J. Oral Pathol. Med. 2008, 37, 389–394. [Google Scholar] [CrossRef]
- Kyle, P.R.; Lemming, O.M.; Timmerby, N.; Søndergaard, S.; Andreasson, K.; Bech, P. The Validity of the Different Versions of the Hamilton Depression Scale in Separating Remission Rates of Placebo and Antidepressants in Clinical Trials of Major Depression. J. Clin. Psychopharmacol. 2016, 36, 453–456. [Google Scholar] [CrossRef]
- Carneiro, A.M.; Fernandes, F.; Moreno, R.A. Hamilton depression rating scale and montgomery–asberg depression rating scale in depressed and bipolar I patients: Psychometric properties in a Brazilian sample. Health Qual. Life Outcomes 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.E.; Zolnoun, D.; Steege, J.F.; Rinaldi, K.L.; Leserman, J. Open-label trial of lamotrigine focusing on efficacy in vulvodynia. J Reprod. Med. 2009, 54, 171–178. [Google Scholar] [PubMed]
- Donham, G.W.; Ludenia, K. Cross-validation of the State-Trait Anxiety Inventory with an alcoholic population. J. Clin. Psychol. 1984, 40, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Calabria, E.; Pecoraro, G.; D′Aniello, L.; Aria, M.; Marenzi, G.; Sammartino, P.; Mignogna, M.D.; Adamo, D. The use of self-report questionnaires in an analysis of the multidimensional aspects of pain and a correlation with the psychological profile and quality of life in patients with burning mouth syndrome: A case-control study. J. Oral Rehabil. 2022, 49, 890–914. [Google Scholar] [CrossRef]
- Zitser, J.; Allen, I.E.; Falgàs, N.; Le, M.M.; Neylan, T.C.; Kramer, J.H.; Walsh, C.M. Pittsburgh Sleep Quality Index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS ONE 2022, 17, e0270095. [Google Scholar] [CrossRef]
- Freedland, K.E.; Steinmeyer, B.C.; Carney, R.M.; Rubin, E.H.; Rich, M.W. Use of the PROMIS® Depression scale and the Beck Depression Inventory in patients with heart failure. Health Psychol. 2019, 38, 369–375. [Google Scholar] [CrossRef]
- Choi, Y.; Mayer, T.G.; Williams, M.J.; Gatchel, R.J. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014, 14, 1175–1182. [Google Scholar] [CrossRef]
- Chan, C.Y.Y.; Tsang, H.H.L.; Lau, C.S.; Chung, H.Y. Prevalence of depressive and anxiety disorders and validation of the Hospital Anxiety and Depression Scale as a screening tool in axial spondyloarthritis patients. Int. J. Rheum. Dis. 2017, 20, 317–325. [Google Scholar] [CrossRef]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncol. 2018, 23, 97–104. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Meader, N.; Symonds, P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: A meta-analysis. J. Affect. Disord. 2010, 126, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Verzak, Ž.; Matijević, M.; Včev, A.; Siber, S.; Musić, L.; Carek, A. Anxiety and Depression Scores in Patients with Burning Mouth Syndrome. Psychiatr. Danub. 2018, 30, 466–470. [Google Scholar] [CrossRef]
- Malik, R.; Goel, S.; Misra, D.; Panjwani, S.; Misra, A. Assessment of anxiety and depression in patients with burning mouth syndrome: A clinical trial. J. Midlife Health 2012, 3, 36–39. [Google Scholar] [CrossRef]
- Burns, J.W.; Day, M.A.; Thorn, B.E. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl. Behav. Med. 2012, 2, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, S.; Chen, G. Assessing the Psychometric Properties of the Chinese Version of Ten-Item Personality Inventory (TIPI) Among Medical College Students. Psychol. Res. Behav. Manag. 2022, 15, 1247–1258. [Google Scholar] [CrossRef]
- Nunes, A.; Limpo, T.; Lima, C.F.; Castro, S.L. Short Scales for the Assessment of Personality Traits: Development and Validation of the Portuguese Ten-Item Personality Inventory (TIPI). Front. Psychol. 2018, 9, 461. [Google Scholar] [CrossRef]
- Thørrisen, M.M.; Sadeghi, T.; Wiers-Jenssen, J. Internal Consistency and Structural Validity of the Norwegian Translation of the Ten-Item Personality Inventory. Front. Psychol. 2021, 12, 723852. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.T.H.; Watanabe, M.; Suga, T.; Hong, C.; Takao, C.; Takenoshita, M.; Motomura, H.; Toyofuku, A. Personality Traits in Burning Mouth Syndrome Patients With and Without a History of Depression. Front. Psychiatry 2021, 12, 659245. [Google Scholar] [CrossRef]
- Darnall, B.D.; Sturgeon, J.A.; Cook, K.F.; Taub, C.J.; Roy, A.; Burns, J.W.; Sullivan, M.; Mackey, S.C. Development and Validation of a Daily Pain Catastrophizing Scale. J. Pain 2017, 18, 1139–1149. [Google Scholar] [CrossRef]
- Cano, A.; Leonard, M.T.; Franz, A. The significant other version of the Pain Catastrophizing Scale (PCS-S): Preliminary validation. Pain 2005, 119, 26–37. [Google Scholar] [CrossRef]
- Rogulj, A.A.; Richter, I.; Brailo, V.; Krstevski, I.; Boras, V.V. Catastrophizing in Patients with Burning Mouth Syndrome. Acta Stomatol. Croat. 2014, 48, 109–115. [Google Scholar] [PubMed]
- Walker, N.A.; Sunderram, J.; Zhang, P.; Lu, S.-E.; Scharf, M.T. Clinical utility of the Epworth sleepiness scale. Sleep Breath. 2020, 24, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Sap-Anan, N.; Pascoe, M.; Wang, L.; Grigg-Damberger, M.M.; Andrews, N.D.; Foldvary-Schaefer, N. The Epworth Sleepiness Scale in epilepsy: Internal consistency and disease-related associations. Epilepsy Behav. 2021, 121, 108099. [Google Scholar] [CrossRef] [PubMed]
- Frohnhofen, H.; Popp, R.; Willmann, V.; Heuer, H.C.; Firat, A. Feasibility of the Epworth Sleepiness Scale in a sample of geriatric in-hospital patients. J. Physiol. Pharmacol. 2009, 60 (Suppl. S5), 45–49. [Google Scholar]
- Damiani, M.F.; Quaranta, V.N.; Falcone, V.A.; Gadaleta, F.; Maiellari, M.; Ranieri, T.; Fanfulla, F.; Carratù, P.; Resta, O. The Epworth Sleepiness Scale: Conventional self vs physician administration. Chest 2013, 143, 1569–1575. [Google Scholar] [CrossRef]
- Adamo, D.; Sardella, A.; Varoni, E.; Lajolo, C.; Biasotto, M.; Ottaviani, G.; Vescovi, P.; Simonazzi, T.; Pentenero, M.; Ardore, M.; et al. The association between burning mouth syndrome and sleep disturbance: A case–control multicentre study. Oral Dis. 2018, 24, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Kirmizigil, B.; Demiralp, C. Effectiveness of functional exercises on pain and sleep quality in patients with primary dysmenorrhea: A randomized clinical trial. Arch. Gynecol. Obstet. 2020, 302, 153–163. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chon, S. Burning mouth syndrome in postmenopausal women with self-reported sleep problems. Cranio® 2020, 38, 221–232. [Google Scholar] [CrossRef]
- Lee, G.-S.; Kim, H.-K.; Kim, M.-E. Relevance of sleep, pain cognition, and psychological distress with regard to pain in patients with burning mouth syndrome. Cranio® 2022, 40, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Lucero-Berdugo, M.; Castillo-Felipe, C.; Zamora Lavella, C.; Ferrandez-Pujante, A.; Pons-Fuster, A. Assessment of self-reported sleep disturbance and psychological status in patients with burning mouth syndrome. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1285–1290. [Google Scholar] [CrossRef]





| Code | Classes |
|---|---|
| 1 | Orofacial pain attributed to disorders of dentoalveolar and anatomically related structures |
| 2 | Myofascial orofacial pain |
| 3 | Temporomandibular joint pain |
| 4 | Orofacial pain attributed to lesion or disease of the cranial nerves |
| 5 | Orofacial pains resembling presentations of primary headaches |
| 6 | Idiopathic orofacial pain |
| 7 | Psychosocial assessment of patients with orofacial pain |
| Topic | References | |
|---|---|---|
| 4.1 | Pain Intensity Assessment | [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] |
| 4.2 | Pain Localization Assessment | [49,50,51,52,53] |
| 4.3 | Pain Quality Assessment | [54,55,56,57,58,59] |
| 4.4 | Pain Behavior Assessment | [60,61,62,63,64,65,66,67,68,69] |
| 4.5 | Quality of Life Assessment | [70,71,72,73,74,75,76,77,78,79,80] |
| 4.6 | Psychological Assessment | [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| 4.7 | Sleep Disorder Assessment | [102,103,104,105,106,107,108,109,110] |
| Random Sequence Generation | Allocation Concealement | Blinding | |
|---|---|---|---|
| Treister et al., 2019 [16] |  |  |  |
| Shafshak et al., 2021 [17] |  |  |  |
| Kendrick et al., 2005 [18] |  |  |  |
| Taddio et al., 2009 [19] |  |  |  |
| Todd et al., 2017 [20] |  |  |  |
| Closs et al., 2004 [21] |  |  |  |
| Lewinson et al., 2013 [22] |  |  |  |
| Ruskin et al., 2014 [23] |  |  |  |
| Wikstrom et al., 2018 [24] |  |  |  |
| Alghadir et al., 2018 [25] |  |  |  |
| Jenkins et al., 2009 [26] |  |  |  |
| Hicks et al., 2001 [27] |  |  |  |
| Suraseranivongse et al., 2005 [28] |  |  |  |
| Sun et al., 2015 [29] |  |  |  |
| Gulur et al., 2009 [30] |  |  |  |
| Fadayevatan et al., 2019 [31] |  |  |  |
| Lee et al., 2015 [32] |  |  |  |
| Girandeau et al., 2004 [33] |  |  |  |
| Ferreira-Valente et al., 2011 [34] |  |  |  |
| Thong et al., 2018 [35] |  |  |  |
| Miró et al., 2016 [36] |  |  |  |
| Malara et al., 2016 [37] |  |  |  |
| Ersek et al., 2010 [38] |  |  |  |
| Paulson-Conger et al., 2011 [39] |  |  |  |
| De-Figuerido et al., 2020 [40] |  |  |  |
| Tran et al., 2023 [41] |  |  |  |
| Odai et al., 2015 [42] |  |  |  |
| Shah et al., 2012 [43] |  |  |  |
| Khatri et al., 2012 [44] |  |  |  |
| Versloot et al., 2004 [45] |  |  |  |
| Felipak et al., 2020 [46] |  |  |  |
| Daher et al., 2015 [47] |  |  |  |
| Senirkentli et al., 2021 [48] |  |  |  |
| Mendonça et al., 2018 [49] |  |  |  |
| Aibel et al., 2023 [50] |  |  |  |
| Elson et al., 2011 [51] |  |  |  |
| Adamo et al., 2020 [52] |  |  |  |
| Sevrain et al., 2015 [53] |  |  |  |
| Melzack et al., 1985 [54] |  |  |  |
| Kachooei et al., 2015 [55] |  |  |  |
| Fontana Carvalho et al., 2020 [56] |  |  |  |
| Renovato França et al., 2010 [57] |  |  |  |
| Dworkin et al., 2015 [58] |  |  |  |
| Erdogan et al., 2019 [59] |  |  |  |
| Lewandowski et al., 2009 [60] |  |  |  |
| Vertsberger et al., 2022 [61] |  |  |  |
| Karoly et al., 2014 [62] |  |  |  |
| Gruszka et al., 2019 [63] |  |  |  |
| Mitra et al., 2020 [64] |  |  |  |
| Delgado et al., 2021 [65] |  |  |  |
| Gomarverdi et al., 2019 [66] |  |  |  |
| Ruscheweyh et al., 2012 [67] |  |  |  |
| Sellers et al., 2020 [68] |  |  |  |
| Bell et al., 2018 [69] |  |  |  |
| Heary et al., 2022 [70] |  |  |  |
| Müller et al., 2017 [71] |  |  |  |
| Kwan et al., 2016 [72] |  |  |  |
| Kishi et al., 2005 [73] |  |  |  |
| Campos et al., 2021 [74] |  |  |  |
| Omara et al., 2021 [75] |  |  |  |
| Musskopf et al., 2018 [76] |  |  |  |
| Yule et al., 2015 [77] |  |  |  |
| Serrano et al., 2022 [78] |  |  |  |
| Chana et al., 2021 [79] |  |  |  |
| López-Jornet et al., 2008 [80] |  |  |  |
| Kyle et al., 2016 [81] |  |  |  |
| Munhoz Carneiro et al., 2015 [82] |  |  |  |
| Meltzer-Brody et al., 2009 [83] |  |  |  |
| Donham et al., 1984 [84] |  |  |  |
| Canfora et al., 2022 [85] |  |  |  |
| Zitser et al., 2022 [86] |  |  |  |
| Freedland et al., 2019 [87] |  |  |  |
| Choi et al., 2014 [88] |  |  |  |
| Chan et al., 2017 [89] |  |  |  |
| Nipp et al., 2018 [90] |  |  |  |
| Silva et al., 2017 [91] |  |  |  |
| Sikora et al., 2018 [92] |  |  |  |
| Malik et al., 2012 [93] |  |  |  |
| Burns et al., 2012 [94] |  |  |  |
| Shi et al., 2022 [95] |  |  |  |
| Nunes et al., 2018 [96] |  |  |  |
| Thørrisen et al., 2021 [97] |  |  |  |
| Huyan et al., 2021 [98] |  |  |  |
| Darnall et al., 2017 [99] |  |  |  |
| Cano et al., 2005 [100] |  |  |  |
| Roguli et al., 2014 [101] |  |  |  |
| Walker et al., 2020 [102] |  |  |  |
| Sap-Anan et al., 2021 [103] |  |  |  |
| Frohnhofen et al., 2009 [104] |  |  |  |
| Damiani et al., 2013 [105] |  |  |  |
| Adamo et al., 2018 [106] |  |  |  |
| Kirmizigil et al., 2020 [107] |  |  |  |
| Lee et al., 2020 [108] |  |  |  |
| Lee et al., 2022 [109] |  |  |  |
| López-Jornet et al., 2014 [110] |  |  |  |
| Aspect | Meaning | Assessment Tools |
|---|---|---|
| Intensity | Related to stress, anxiety, and absent pain coping mechanisms | VAS, NRS, VRS, FPS, RAS, PAINAD, DDQ |
| Localization | Allows for a targeted pain assessment and therapeutic response | Pain maps, MPQ |
| Quality | Permits doctors to distinguish the sensorial and the emotional–experiential components of pain | MPQ |
| Behavioral implications | Related to low QoL perception and PI increase | Clinical evaluation, pain diary, BPS, PSQ |
| Quality of life | Predictor of future pain | SF-36, OHIP |
| Psychological aspects | Related to perceived PI increase and low QoL perception | HAM-A, HAM-D, STAI, BDI, HADS, TIPI, PCS |
| Sleep disturbances | Related to mood worsening, higher stress and disability, and pain tolerance lowering | ESS, PSQI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; Pellegrini, M.; Pulicari, F.; Ghizzoni, M.; Modugno, F.P.; Spadari, F. Pain Assessment in Oral Medicine through Its Different Dimensions: A Comprehensive Review. Dent. J. 2023, 11, 246. https://doi.org/10.3390/dj11110246
Scribante A, Pellegrini M, Pulicari F, Ghizzoni M, Modugno FP, Spadari F. Pain Assessment in Oral Medicine through Its Different Dimensions: A Comprehensive Review. Dentistry Journal. 2023; 11(11):246. https://doi.org/10.3390/dj11110246
Chicago/Turabian StyleScribante, Andrea, Matteo Pellegrini, Federica Pulicari, Martina Ghizzoni, Francesco Paolo Modugno, and Francesco Spadari. 2023. "Pain Assessment in Oral Medicine through Its Different Dimensions: A Comprehensive Review" Dentistry Journal 11, no. 11: 246. https://doi.org/10.3390/dj11110246