Time-to-Treatment of Oral Cancer and Potentially Malignant Oral Disorders: Findings in Malaysian Public Healthcare
Abstract
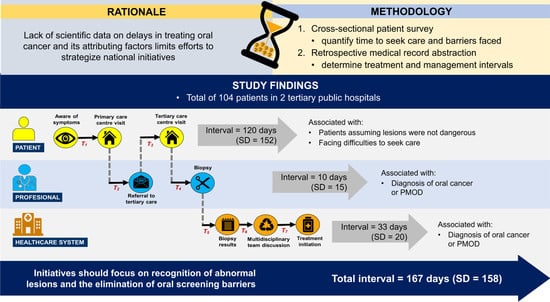
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Defining Time-to-Treatment
2.3. Patient Survey
2.4. Medical Record Abstraction
2.5. Statistical Analysis
3. Results
3.1. Time-to-Treatment
3.2. Factors Influencing the Patient Interval
3.3. Factors Influencing Professional and System Intervals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Rao, S.V.K.; Mejia, G.; Roberts-Thomson, K.; Logan, R. Epidemiology of oral cancer in Asia in the past decade-an update (2000-2012). Asian Pac. J. Cancer Prev. 2013, 14, 5567–5577. [Google Scholar]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow (accessed on 6 June 2021).
- Warnakulasuriya, S.; Greenspan, J.S. Textbook of Oral Cancer: Prevention, Diagnosis and Management; Springer: New York, NY, USA, 2020; Volume 1, pp. 1–452. [Google Scholar]
- Brocklehurst, P.; Kujan, O.; O’Malley, L.A.; Ogden, G.; Shepherd, S.; Glenny, A.M. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst. Rev. 2013, 11, CD004150. [Google Scholar]
- Ministry of Health Malaysia. National oral Health Plan for Malaysia 2011–2020; Ministry of Health Malaysia: Putrajaya, Malaysia, 2011. [Google Scholar]
- Azizah, A.; Hashimah, B.; Nirmal, K.; Siti Zubaidah, A.; Puteri, N.; Nabihah, A.; Sukumaran, R.; Balqis, B.; Nadia, S.; Sharifah, S.; et al. Malaysian National Cancer Registry Report 2012–2016: Malaysia Cancer Statistic, Data and Figure; National Cancer Institute: Putrajaya, Malaysia, 2019. [Google Scholar]
- Noonan, B. Understanding the reasons why patients delay seeking treatment for oral cancer symptoms from a primary health care professional: An integrative literature review. Eur. J. Oncol. Nurs. 2014, 18, 118–124. [Google Scholar]
- Gajendra, S.; Cruz, G.D.; Kumar, J.V. Oral cancer prevention and early detection: Knowledge, practices, and opinions of oral health care providers in New York State. J. Cancer Educ. 2006, 21, 157–162. [Google Scholar]
- Ng, C.-W. Universal Health Coverage Assessment: Malaysia; Global Network for Health Equity (GNHE): Ottawa, ON, Canada, 2015. [Google Scholar]
- Yu, T.; Wood, R.E.; Tenenbaum, H.C.; Perio, D. Delay in diagnosis of head and neck cancers. J. Can. Dent. Assoc. 2008, 74, 198–203. [Google Scholar]
- Saka-Herrán, C.; Jané-Salas, E.; Mari-Roig, A.; Estrugo-Devesa, A.; López-López, J. Time-to-treatment in oral cancer: Causes and implications for survival. Cancers 2021, 13, 1321. [Google Scholar]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Warnakulasuriya, S.; Johnson, N.W.; Van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral. Pathol. Med. 2007, 36, 575–580. [Google Scholar]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer. 2012, 106, 1262–1267. [Google Scholar]
- Olesen, F.; Hansen, R.; Vedsted, P. Delay in diagnosis: The experience in Denmark. Br. J. Cancer. 2009, 101, S5–S8. [Google Scholar]
- Ng, A.; Firouz, A.; Khalidi, J.; Muhtar, M.; Tumin, S.; Man, T. The State of Households 2018: Different Realities; Khazanah Research Institute: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Xiao, R.; Ward, M.C.; Yang, K.; Adelstein, D.J.; Koyfman, S.A.; Prendes, B.L.; Burkey, B.B. Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: A mechanism for increased mortality. Cancer 2018, 124, 1400–1414. [Google Scholar]
- Lopez-Cedrún, J.L.; Varela-Centelles, P.; Otero-Rico, A.; Vázquez-Mahía, I.; Seoane, J.; Castelo-Baz, P.; Seoane-Romero, J. Overall time interval (“total diagnostic delay”) and mortality in symptomatic oral cancer: A U-shaped association. Oral Oncol. 2020, 104, 104626. [Google Scholar]
- NICE. Suspected Cancer: Recognition and Referral (NG12); NICE: London, UK, 2015. [Google Scholar]
- Khoo, S.P.; Shanmuhasuntharam, P.; Mustafa, W.M.; Tay, K.; Latif, A.; Nair, S. Delay in diagnosis of oral cancer in Malaysia: A study of five centres. Ann. Dent. UM. 1996, 3, 1–4. [Google Scholar]
- Varela-Centelles, P.; Seoane, J.; Lopez-Cedrun, J.; Fernandez-Sanroman, J.; García-Martin, J.M.; Takkouche, B.; Alvarez-Novoa, P.; Seoane-Romero, J.M. The length of patient and primary care time interval in the pathways to treatment in symptomatic oral cancer. A quantitative systematic review. Clin. Otolaryngol. 2018, 43, 164–171. [Google Scholar]
- Stefanuto, P.; Doucet, J.-C.; Robertson, C. Delays in treatment of oral cancer: A review of the current literature. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2014, 117, 424–429. [Google Scholar]
- Da Conceição, M.G.D.; Emmerick, I.C.M.; Figueiró, A.C.; Luiza, V.L. Oral cancer patient’s profile and time to treatment initiation in the public health system in Rio de Janeiro, Brazil. BMC Health Serv. Res. 2021, 21, 1–8. [Google Scholar]
- Güneri, P.; Epstein, J.B. Late stage diagnosis of oral cancer: Components and possible solutions. Oral Oncol. 2014, 50, 1131–1136. [Google Scholar]
- Ministry of Health Malaysia. Malaysia National Health Accounts: Health Expenditure Report 1997–2017; Ministry of Health Malaysia: Putrajaya, Malaysia, 2019; Volume 1, p. 120. [Google Scholar]
- Bohari, N.F.M.; Kruger, E.; John, J.; Tennant, M. Analysis of dental services distribution in Malaysia: A geographic information systems–based approach. Int. Dent. J. 2019, 69, 223–229. [Google Scholar]
- Onizawa, K.; Nishihara, K.; Yamagata, K.; Yusa, H.; Yanagawa, T.; Yoshida, H. Factors associated with diagnostic delay of oral squamous cell carcinoma. Oral Oncol. 2003, 39, 781–788. [Google Scholar]
- Chen, Y.; Huang, H.; Lin, L.; Lin, C. Primary oral squamous cell carcinoma: An analysis of 703 cases in southern Taiwan. Oral Oncol. 1999, 35, 173–179. [Google Scholar]
- Azhar, N.; Doss, J.G. Health-seeking behaviour and delayed presentation of oral cancer patients in a developing country: A qualitative study based on the self-regulatory model. Asian Pac. J. Cancer. Prev. 2018, 19, 2935. [Google Scholar]
- Awojobi, O.; Scott, S.E.; Newton, T. Patients’ perceptions of oral cancer screening in dental practice: A cross-sectional study. BMC Oral Health 2012, 12, 1–9. [Google Scholar]
- Scott, S.E.; Grunfeld, E.A.; McGurk, M. Patient’s delay in oral cancer: A systematic review. Community Dent. Oral Epidemiol. 2006, 34, 337–343. [Google Scholar]
- Raman, S.; Shafie, A.A.; Abraham, M.T.; Shim, C.K.; Maling, T.H.; Rajendran, S.; Cheong, S.C. Provider cost of treating oral potentially malignant disorders and oral cancer in Malaysian public hospitals. PLoS ONE 2021, 16, e0251760. [Google Scholar]

| Interval 1 | Process | Duration (Days) |
|---|---|---|
| Patient | T1 | From the time the patient first became aware of symptoms to the first visit to primary care |
| Professional | T2 | From the first visit to primary care to the first referral to a specialist |
| Patient | T3 | From the appointment date to actual attendance at the specialist clinic |
| Professional | T4 | From the first exam by a specialist to the period a biopsy was first performed |
| System | T5 | From the first biopsy taken to the reporting of biopsy results |
| System | T6 | From the reporting of the biopsy results to the assignment of a treatment plan by a multidisciplinary oral cancer team |
| System | T7 | From the assignment of a treatment plan to the initiation of definitive treatment (first day of radiotherapy, chemotherapy, surgery, or oral/topical treatment) |
| Characteristic | PMOD (n = 52) Freq (%) | Cancer (n = 52) Freq (%) | p-Value 1 | |
|---|---|---|---|---|
| Age | <60 | 25 (48.1) | 26 (50.0) | 0.844 |
| >60 | 27 (51.9) | 26 (50.0) | ||
| Gender | Male | 18 (34.6) | 24 (46.2) | 0.230 |
| Female | 34 (65.4) | 28 (53.8) | ||
| Race | Malay | 14 (26.9) | 7 (13.5) | 0.110 |
| Chinese | 6 (11.5) | 14 (26.9) | ||
| Indian | 27 (51.9) | 24 (46.1) | ||
| Indigenous | 5 (9.6) | 7 (13.5) | ||
| Location | Urban | 26 (50.0) | 23 (44.2) | 0.556 |
| Rural | 26 (50.0) | 29 (55.8) | ||
| Education | None/Primary | 18 (34.6) | 30 (57.7) | 0.018 |
| Secondary/Tertiary | 34 (65.4) | 22 (42.3) | ||
| Occupation | Not employed | 21 (40.4) | 23 (44.2) | 0.691 |
| Employed/Retired | 31 (59.6) | 29 (55.8) | ||
| Household income | ≤MYR 4360 | 47 (90.4) | 46 (88.5) | 0.750 |
| >MYR 4360 | 5 (9.6) | 6 (11.5) | ||
| Anatomic site | Buccal mucosa | 32 (61.5) | 25 (48.1) | 0.408 |
| Tongue | 13 (25.0) | 14 (26.9) | ||
| Others 2 | 7 (13.5) | 13 (25.0) | ||
| Interval | Duration (Days) | p-Value 1 | |||
|---|---|---|---|---|---|
| PMOD (n = 52) | Cancer (n = 52) | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Patient | 111 (143) | 64 (15–120) | 128 (155) | 61 (21–183) | 0.7616 |
| T1: Symptom to the first primary care visit | 106 (140) | 60 (15–105) | 130 (156) | 60 (23–180) | 0.5075 |
| T3: Specialist appointment to actual clinic attendance | 5 (10) | 1 (0–4) | 1 (3) | 0 (0–2) | 0.0564 |
| Professional | 15 (17) | 8 (2–20) | 4 (8) | 1 (0–6) | 0.0001 |
| T2: First primary visit to specialist referral | 7 (12) | 1 (0–8) | 2 (7) | 0 (0–1) | 0.0046 |
| T4: Specialist visit to biopsy | 9 (14) | 5 (0–11) | 2 (4) | 0 (0–1) | 0.0006 |
| System | 25 (18) | 21 (11–37) | 42 (19) | 41 (29–50) | 0.0001 |
| T5: First biopsy to biopsy results | 11 (6) | 10 (6–16) | 9 (5) | 8 (6–12) | 0.1211 |
| T6: Biopsy results to treatment plan | 11 (10) | 9 (1–16) | 19 (12) | 15 (9–29) | 0.0009 |
| T7: Treatment plan to treatment initiation | 6 (12) | 0 (0–13) | 14 (11) | 11 (6–20) | 0.0001 |
| TOTAL delay | 157 (154) | 109 (68–171) | 176 (163) | 95 (67–230) | 0.9626 |
| Author | Year | Population | Interval Period (in Days) | ||
|---|---|---|---|---|---|
| Patient | Professional | Healthcare | |||
| Study findings | 2021 | Malaysia | 120 | 10 | 33 |
| Khoo et al. [21] | 1996 | Malaysia | 202 | 72 1 | - |
| Varela-Centelles et al. [22] | 2017 | Europe, USA, India, Australia, Japan, Argentina, Iran | 80 | 16 | 59 |
| Saka-Herrán et al. [12] | 2021 | USA, Germany, China, Europe, Iran, India, Australia, Japan, Argentina, Canada, Denmark | 48–168 | 14–90 2 | 29–57 2 |
| Stefanuto et al. [23] | 2014 | Germany, USA, Canada, UK, Thailand, Japan, the Netherlands | 105–162 | 98–147 | |
| Available recommendations | |||||
| NICE Guideline (NG12) [20] | 2021 | United Kingdom | 0–21 3 | 14 4 | - |
| Brazil Federal Law [24] | 2012 | Brazil | - | 30 5 | 60 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, S.; Shafie, A.A.; Abraham, M.T.; Kiong, S.C.; Maling, T.H.; Rajendran, S.; Cheong, S.C. Time-to-Treatment of Oral Cancer and Potentially Malignant Oral Disorders: Findings in Malaysian Public Healthcare. Dent. J. 2022, 10, 199. https://doi.org/10.3390/dj10110199
Raman S, Shafie AA, Abraham MT, Kiong SC, Maling TH, Rajendran S, Cheong SC. Time-to-Treatment of Oral Cancer and Potentially Malignant Oral Disorders: Findings in Malaysian Public Healthcare. Dentistry Journal. 2022; 10(11):199. https://doi.org/10.3390/dj10110199
Chicago/Turabian StyleRaman, Sivaraj, Asrul Akmal Shafie, Mannil Thomas Abraham, Shim Chen Kiong, Thaddius Herman Maling, Senthilmani Rajendran, and Sok Ching Cheong. 2022. "Time-to-Treatment of Oral Cancer and Potentially Malignant Oral Disorders: Findings in Malaysian Public Healthcare" Dentistry Journal 10, no. 11: 199. https://doi.org/10.3390/dj10110199