1. Introduction
Titanium dioxide (TiO
2) is a wide bandgap semiconductor available in the form of three crystalline polymorphs: rutile (tetragonal), anatase (tetragonal), and brookite (orthorhombic). TiO
2 is a widely studied material due to its very attractive physical properties, such as long-term stability, oxidative ability, wide indirect band gap [
1], low cost and low biological toxicity. These qualities make it a promising material for photoelectric applications, such as semiconductor electrodes in solar cells [
2], photo-catalysis [
3], sensors [
4], adsorption [
5], biosensors [
6,
7,
8], cancer therapy [
9,
10], and UV detectors [
11,
12].
Thin film materials are practical and attracted extensive interdisciplinary research interests between physics, chemistry, and electronics [
13,
14]. A functional material in the form of a porous film solidly bonded to cheap substrates (steel or polymers) allows it to function effectively and practically, this is important particularly for large-scale industrial applications. Numerous applications involve, for example, optical films for dye-sensitized solar cells [
15], as electrodes in electrochemistry [
16], photo-electrocatalysis [
17], and photo-catalysis [
18], and many others [
19].
A large number of processing techniques were developed for the deposition of TiO
2 films. TiO
2 thick films are fabricated by a colloidal processing route (e.g., electrophoretic deposition) [
20,
21] and hydrothermal methods [
22,
23]. Sol-gel processing is also used to fabricate TiO
2 thin films [
24,
25,
26], however, it presents film cracking and peeling [
27], and there is also a thickness limitation for each layer (~1 µm). Several vapor processing techniques, (i.e., sputtering [
28,
29]), chemical vapor deposition [
30,
31,
32], spray pyrolysis [
33,
34], and physical vapor deposition (PVD) [
35,
36]), were applied to fabricate TiO
2 thin films.
The PVD refers to the processes of deposition of thin films and nanostructures through evaporation of solid precursors in their vapor phase by physical approaches, followed by condensation of the vapor phase on substrates. The whole process consists of three stages: (1) evaporation of the solid source, (2) vapor phase transport from the source to the substrates, and (3) vapor condensation on the substrates [
37]. Common PVD techniques include laser ablation [
38,
39], sputtering [
40,
41], and cathodic arc [
42,
43,
44,
45]. In the PVD techniques the structure of TiO
2 films and their properties depend on the deposition method and on the substrate temperatures during film growth and the energy of the ions involved.
In the last two decades, great progress was achieved in various applications of TiO
2, however, within the TiO
2 structure the photo-excited electrons and holes rapidly recombine, thus diminishing the effectiveness of photo-catalysis [
46]. Several approaches were proposed to amend these difficulties; one of the effective modifications is the doping with noble metals in TiO
2, where hetero-structures and new interfaces are formed that improve the photo-catalytic efficiency. Localized surface plasmon resonance (LSPR) on noble metal surfaces, which arises from the collective oscillations of electrons near them, enhances the interaction between catalysts and light, and is explained as the mechanism responsible for affecting the photo-catalytic efficiency [
47]. So far, Pt [
48], Au [
49], Ag [
50], and Cu [
51], were added to TiO
2. Among the metals, Ag is widely used because it is comparatively cheap, showing a suitable work function.
In particular, heterogeneous catalysis using TiO
2 as a photocatalyst is an attractive method for the decomposition of various undesirable pollutants [
52,
53]. As persistent organic pollutants (POPs) are a group of highly toxic chemicals for humans and the environment, several governments in the world signed the Stockholm Convention, which came into force in 2004 and established the elimination of twelve priority POPs [
54]. Some examples of these POP-type compounds are pesticides, herbicides, toxic dyes used in the textile industry, and organochlorine insecticides, among others. Organic dyes require special attention, as they are shown to be highly water soluble, toxic, carcinogenic, and mutagenic to humans, animals, and aquatic life [
55]. The textile industry in its process uses dyes with complex and persistent structures, such as rhodamine B (RhB), which is a cationic dye of the xanthene class, highly soluble in water, and widely used as a textile dye and in food products. It is also a well-known fluorescent tracer and biomarker [
56,
57,
58,
59]. Due to its wide use, its degradation mechanism was studied previously; the degradation products are known [
60,
61] and can be easily followed by spectrophotometry.
The concentration of toxic materials and infectious microorganisms in the natural resources of drinking water is constantly increasing, causing severe environmental pollution. The traditional chemical methods for the disinfection of drinking water have limitations due to their costs for undeveloped nations. Furthermore, a generation of harmful disinfection byproducts associated with the chemical disinfection processes, such as chlorination, is a source of rising concern [
62].
Table 1 shows some works on dye degradation and disinfection by photocatalysis using TiO
2 and TiO
2 with Ag.
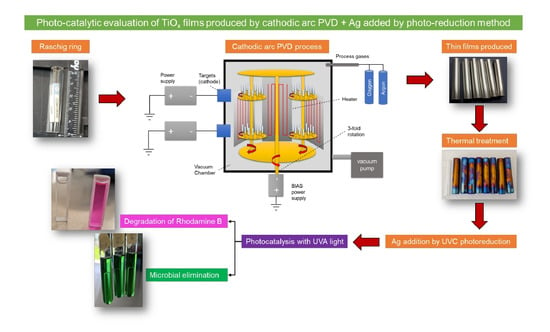
For such reasons in this research, a series of TiO2 coatings was synthesized by the cathodic arc-PVD deposition method on commercial borosilicate Raschig rings (Morelia, Mexico). The effect of the O flow during deposition on the TiOx characteristics was analyzed. A thermal treatment is explored to increase the amount of oxygen on the surface of the coating, and Ag was added into the film by the photo-reduction method under UVC light. The photo-catalytic activity of these TiOx PVD functional coatings is evaluated in model reactions.
3. Materials and Methods
3.1. Synthesis of Thin Films by PVD on Borosilicate Raschig Rings
Commercial borosilicate Raschig rings with Ø = 10 mm, length = 50 mm, and wall thickness = 1.5 mm were used as substrates for TiO
x deposition. First, the substrate rings were cleaned in an ultrasonic, using Elmasonic X-tra equipment (Singen, Germany) with an operating frequency of 45 KHz in ethanol for 15 min at room temperature. Then, they were dried and fixed in a 3-axis rotating planetary system for coating deposition, see left image in
Figure 1. The rotating planetary system was introduced inside the coating chamber of the DOMINO mini semi-industrial equipment from OERLIKON (Bergisch Gladbach, Germany), where the TiO
x coating was deposited applying the cathodic arc method, see the coated Rasching rings in the middle and right images in
Figure 13.
Firstly, the rings are heated at ~450 °C using a 9 kW heating system and applying a continuous vacuum. The heating provides cleaning by evaporating residual moisture from the surface of the borosilicate rings. Before the coating step, the rings’ surface was cleaned with Ar+ ions produced through the arc enhanced glow discharge (AEGD) process. During the coating of the TiOx, a reaction takes place between the metallic Ti ions generated by erosion of the cathode material and the O ions. High purity gases are used for this process, oxygen (O2) with a purity of 99.998%, and Ar with a purity of 99.999%. The Ar and O2 flow rates were varied for each set of rings, and three sets of Ar/O2 ratios (440/60, 400/100, and 300/100) were selected and 11 Raschig rings were deposited during each coating run. The deposition time was maintained for 2 h by applying 80 V bias at a frequency of 20/80 KHz and a current of 150 A. Lastly, the coated samples were cooled inside the PVD chamber for ~90 min.
The ring sets were characterized by field emission scanning electron microscopy (FE-SEM) for surface analysis, thickness, and morphology of the TiOx films, X-ray energy dispersive spectrometry (EDS) for elemental analysis, and low angle X-ray diffraction (XRD) to identify crystalline phases in the coating. For this purpose, the XRD data were taken in a Rigaku diffractometer and by applying the parallel beam (PB) mode using Cu Kα radiation with a wavelength of 1.540593 Å, angle ω = 1°, an angular range from 20° to 90°, and a step size of 0.02°.
3.2. Heat Treatment after PVD Coating
The Raschig rings coated with the TiO
x systems were heat-treated in a Thermo Scientific Lindberg Blue M furnace (Waltham, MA, USA) at 500 °C for 2 h. Using a heating ramp of 10 °C/min, this treatment was performed under standard conditions and air atmosphere. The heat treatment was performed in order to increase the O content on the surface of the coating according to [
71,
72]. Subsequently, the grazing incidence X-ray diffraction XRD method was applied to analyze the degree of oxidation and diffuse reflectance analysis was performed.
Figure 14 shows the rings after heat treatment.
3.3. Doping of the TiOx with Silver
Silver (Ag) was added to the TiO
x coating to activate it, and for this purpose, the photo-chemical reduction or photo-deposition methodology was applied, where the Ti/TiO
2-coated rings were immersed twice for 5 min in a 1.7 wt. % AgNO
3 aqueous solution with CAS number 7761-88-8. Subsequently, they were irradiated with a UVC source (λ = 254 nm) for 2 h to activate the impregnated Ag [
24,
73]. X-ray diffraction (XRD) and diffuse reflectance analysis were performed after this doping process.
Ag, is used, which is the most promising metal, serving as a conduction band electron attractor, increasing the hole-electron separation, and facilitating electron excitation by the creation of a local electric field [
74,
75]. In this way, e
−/h
+ recombination is avoided. This property is due to the high reduction potential of the Ag
+ ion (Ag
+/ Ag
0) producing metallic Ag on the TiO
2 surface.
3.4. Dye Degradation Experiments
The photocatalytic activity of each set of rings coated with the Ag-doped TiO2 was evaluated in the degradation of rhodamine B (RhB) with CAS number 81-88-9. Firstly, the calibration curve of the rhodamine B dye was performed, taking as reference the wavelength of maximum absorption of the dye of 554 nm. The photocatalytic degradation reactions were carried out in the photocatalytic reactor where its geometry corresponds to a cylinder 90 cm long, with a diameter of 3 ½” made of polyvinyl chloride (PVC) and with a stainless steel reflecting surface. Inside the reactor there is a 20 W TecnoLite F20T8BLB UV-A lamp of short wavelength between 315 and 400 nm, which is inside a quartz tube of DExt = 3.2 cm, a wall thickness of 0.2 cm, and a length of 91.5 cm. The rings are attached to nylon threads from the top cover and are located in the free space between the quartz tube and the stainless steel surface, and the bottom is air inlet through an Elite 799 pump. The reactor operates in batch mode, with a volume of 2 l of a 0.5 ppm rhodamine B solution. In a typical photo-catalytic experiment, the aqueous solution was loaded into the reactor, left in the dark with stirring for 30 min to equilibrate, and then the UV-A lamp was turned on. Aliquots were taken at specific time intervals and analyzed using a BIOBASE BK-UV1800 spectrophotometer (Jinan, China). The obtained data were plotted to determine the reaction order. Subsequently, kinetic parameters, such as reaction rate, constant and half-life time, were calculated; this was done in order to evaluate the photo-catalytic efficiency of each set of TiO2-coated rings. In the reaction system, the following experimental arrangement was carried out in the degradation of rhodamine B: (a) use of immobilized TiO2 in the dark, (b) use of UV light lamp without immobilized TiO2 “photolysis”, (c) use of immobilized TiO2 with UV light lamp “photocatalysis”, and (d) degradation in the dark without catalyst.
3.5. Disinfection Experiments
The coated Ag-doped TiO2 rings were evaluated in the elimination of fecal coliforms. For these, two water matrices were used: one from the secondary treatment effluent (before chlorination process) of the wastewater treatment plant of GEA Ambiental, Morelia, Mexico, and synthetic water prepared in the laboratory.
For the preparation of synthetic water, a sample was taken from the secondary treatment effluent of the GEA Ambiental plant and bacterial growth was performed in MacConkey broth medium. After incubation for 24–48 h at 35 °C, bacterial cells were transferred to 50 mL conical polypropylene centrifuge tubes with flat caps and collected by centrifugation at 4000 rpm for 5 min at room temperature. The supernatant was discarded and the sediment was resuspended in sterile phosphate solution or 0.9% NaCl normal saline. This solution is called microbial stock solution [
76]. To obtain the synthetic water to be used in photocatalytic disinfection, ~ 20 mL of the microbial stock solution is resuspended in 2 L of sterile distilled water and mixed with magnetic stirring.
In a typical experiment, the inoculated aqueous solution was loaded into the reactor, left in the dark with stirring for 30 min to equilibrate, and then exposed to UV-A radiation. An aliquot was extracted at the beginning and end of the process, which was after 4 h. In order to evaluate the percentage of elimination of fecal coliforms, both aliquots were analyzed by means of the microbiological test of the most probable number in multiple tubes (MPN) for fecal coliforms following the Mexican standard NMX-AA-042-SCFI-2015.
The following tests are performed in the reactor in the elimination of fecal coliforms: (a) use of immobilized TiO2 in darkness, (b) use of UV light lamp without immobilized TiO2 “photolysis”, (c) use of immobilized TiO2 with UV light lamp “photocatalysis” and (d) degradation in the darkness without catalyst.
4. Conclusions
Heterogeneous photocatalysis stands out among advanced oxidation processes (AOPs) as a promising and effective biocidal technique, with titanium dioxide (TiO2) being the most common catalyst employed for the purification of aqueous matrices. However, one of the main obstacles to putting it into practice is the separation of photocatalysts and the design of photocatalytic reactors, as most of the synthesized photocatalysts are in the form of powders. Alternatively, TiO2 can be fixed on a solid support, eliminating the need to add a catalyst separation process. In this case, it should be noted that the process offers lower efficiencies due to the smaller surface area of catalysts exposed to light and molecules to be removed.
The Ag-TiOx photocatalyst deposited with 400 Ar/100 O2 degraded ~68.18%, and the catalytic coating deposited with 300 Ar/100 O2 degraded ~68.02% of the rhodamine B dye at a concentration of 0.5 ppm in 2 L volume. In the disinfection, the TiOx 300 Ar/100 O2 coating had a fecal coliform removal of 83.69% and the TiOx 400 Ar/100 O2 coating removed 90.32%.
The results obtained cannot be directly compared with others because each of them used different reaction conditions, such as type of illumination, concentration of TiO
2 and rhodamine B, reactor design, catalyst immobilization method, and coating substrate. All these parameters are factors that have an effect on the kinetics of rhodamine B degradation and bacterial killing. However, they are positive results on rhodamine B degradation for TiO
x-Ag thin films if the results obtained for TiO
2-Ag thin films synthesized with a sol-gel method deposited by dip coating on the Ag substrates and photo-chemical deposition shown in
Table 1 are known.
Targeting the application of fixed photo-catalysts, TiOx coatings were deposited by cathodic arc physical vapor deposition on borosilicate Raschig rings. The deposit was designed for O grading using flow ratios of 440 Ar/60 O2, 400 Ar/100 O2, and 300 Ar/100 O2 in a semi-industrial coater unit. The arc-PVD plasma was stable in all Ar/O2 mixtures.
In the as-coated state, the TiOx catalysts are composed of pure Ti in the α phases. The EDS analysis showed that O and Ti varies in the depth of the coating, suggesting the formation of a system with a low amount of O producing a nonstoichiometric oxide (TiOx). A heat treatment was needed in order to increase the amount of O in the coating and build the crystalline TiO2 rutile phase.
After the thermal treatment, the analysis by UV vis diffuse reflectance spectroscopy indicated that the TiOx catalytic coatings using 400 Ar/100 O2 and 300 Ar/100 O2 are activated under UVA radiation, while the coating 440 Ar/60 O2 absorbs in the visible spectrum.
Silver, confirmed by XRD, was added by the UVC photo-reduction method in the TiOx photo-catalysts deposited using 400 Ar/100 O2 and 300 Ar/100 O2 sccm sets. In this Ag-TiOx photo-catalyst, a decrease in the bandgap energy was observed, thus showing moderate photo-catalytic activity in the degradation of rhodamine B at 0.5 ppm and the removal of fecal coliforms from a secondary treatment effluent and synthetic water.