A Facile and Green Synthesis of Hydrophobic Polydimethylsiloxane Foam for Benzene, Toluene, and Xylene Removal
Abstract
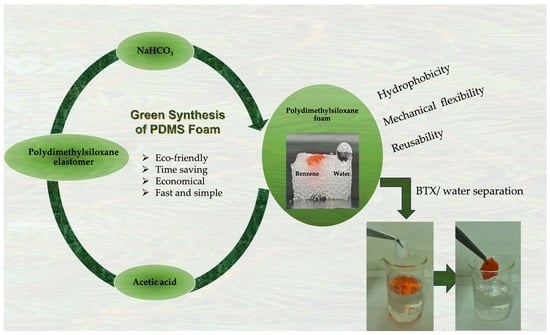
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PDMS Foams
2.3. Characterization
2.4. The Absorption Study
3. Results and Discussion
3.1. Preparation of PDMS Foams
3.2. Characterization of the PDMS Foam
3.2.1. Morphology
Effect of NaHCO3: Acetic Acid Ratios
Effect of Curing Temperature
3.2.2. Measurement of Density, Porosity, and Hydrophobicity
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.4. Mechanical Properties
3.3. Absorption Study
3.3.1. Effect of Preparation Parameters on the Absorption of Benzene
3.3.2. Absorption of BTX
3.3.3. Sorption from the Water’s Surface
| Foam Material | Preparation Method | Density (g cm−3) | Porosity (%) | Pore Volume (μm) | Maximum Compression Strain | Water Contact Angle (°) | Absorption Capacity for BTX (g/g) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| B | T | X | ||||||||
| PDMS | Sugar particle template | 0.18–0.75 | N/A | N/A | N/A | 120–130 | - | 4–5 | - | [37] |
| AuNP/PDMS | Emulsion template | 0.9–1.3 | N/A | 0.97–3.12 | 48–127% | N/A | 3.5 | 4.5 | 6 | [61] |
| PDMS/wax nanocrystal | Sugar cube template | 0.28–0.43 | 56–71 | 50–100 | N/A | 151 | 9 | 13 | 10 | [62] |
| AuNP/PDMS | Emulsion template | N/A | 10–1000 | N/A | N/A | - | 6 | - | [63] | |
| PDMS/GO | Gas foaming | N/A | 65.1–71.6 | 0.1–1000 | 80% | 117.4–138.1 | 9 | [64] | ||
| PDMS | Gas foaming | N/A | N/A | 30–500 | N/A | 139 | - | - | - | [19] |
| PDMS | Sugar particle template | 0.12–1.1 | 43–84 | N/A | 60% | 144 | - | 18.7 | [65] | |
| PDMS | Emulsion template | N/A | 93 | 65.8–1768 | 90% | 145.5 | - | 19 | - | [41] |
| PDMS | Emulsion template | 0.131–0.847 | 8.80–85.6 | 15–5000 | 90% | 115–141 | - | - | - | [44] |
| PDMS/Graphene | Salt template | 0.14 | 80 | N/A | 70% | 140–149 | - | - | - | [66] |
| PDMS/Graphene | Sugar cones template | N/A | N/A | N/A | N/A | 101–126 | - | 6 | - | [67] |
| DMS/CNFs | Sugar cube template | 0.425 | N/A | N/A | 50% | 131–151 | - | 1.4 | - | [68] |
| PDMS | Gas foaming | 0.126–0.348 | 73–88 | 100–1400 | 95% | 110–139 | 7.5 | 7.6 | 7.8 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, C.F.; Wang, P.H.; Zhang, J.W.; Guo, K.Y.; Li, Y.; Xia, Q.Q.; Zhang, G.D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-Step and Green Synthesis of Lightweight, Mechanically Flexible and Flame-Retardant Polydimethylsiloxane Foam Nanocomposites via Surface-Assembling Ultralow Content of Graphene Derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Pan, Z.; Guan, Y.; Liu, Y.; Cheng, F. Facile Fabrication of Hydrophobic and Underwater Superoleophilic Elastic and Mechanical Robust Graphene/PDMS Sponge for Oil/Water Separation. Sep. Purif. Technol. 2021, 261, 118273. [Google Scholar] [CrossRef]
- Kwak, Y.; Kang, Y.; Park, W.; Jo, E.; Kim, J. Fabrication of Fine-Pored Polydimethylsiloxane Using an Isopropyl Alcohol and Water Mixture for Adjustable Mechanical, Optical, and Thermal Properties. RSC Adv. 2021, 11, 18061–18067. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.A.; Brünig, H.; Boldt, R.; Heinrich, G. Morphology Development from Rod-like to Nanofibrillar Structures of Dispersed Poly (Lactic Acid) Phase in a Binary Blend with Poly (Vinyl Alcohol) Matrix along the Spinline. Polymer 2014, 55, 6354–6363. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, B.; Jiang, R.; Cui, Y.; Xu, T.; Zhou, M.; Li, Z. Polydimethylsiloxane/Carbonized Bacterial Cellulose Sponge for Oil/Water Separation. Process Saf. Environ. Prot. 2022, 165, 173–180. [Google Scholar] [CrossRef]
- Masihi, S.; Panahi, M.; Maddipatla, D.; Hanson, A.J.; Bose, A.K.; Hajian, S.; Palaniappan, V.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Highly Sensitive Porous PDMS-Based Capacitive Pressure Sensors Fabricated on Fabric Platform for Wearable Applications. ACS Sens. 2021, 6, 938–949. [Google Scholar] [CrossRef]
- Michel, T.R.; Capasso, M.J.; Cavusoglu, M.E.; Decker, J.; Zeppilli, D.; Zhu, C.; Bakrania, S.; Kadlowec, J.A.; Xue, W. Evaluation of Porous Polydimethylsiloxane/Carbon Nanotubes (PDMS/CNTs) Nanocomposites as Piezoresistive Sensor Materials. Microsyst. Technol. 2019, 4, 1101–1112. [Google Scholar] [CrossRef]
- Xu, B.; Ye, F.; Chen, R.; Luo, X.; Xue, Z.; Li, R.; Chang, G. A Supersensitive Wearable Sensor Constructed with PDMS Porous Foam and Multi-Integrated Conductive Pathways Structure. Ceram. Int. 2023, 49, 4641–4649. [Google Scholar] [CrossRef]
- Tamhane, D.U.; Morarka, A.R. On the Attenuation of Light by a Polydimethylsiloxane (PDMS) Foam and Its Implementation as a Weight Sensor. Mapan J. Metrol. Soc. India 2017, 32, 1–6. [Google Scholar] [CrossRef]
- He, Y.; Lu, X.; Wu, D.; Zhou, M.; He, G.; Zhang, J.; Zhang, L.; Liu, H.; Liu, C. CNT/PDMS Conductive Foam-Based Piezoresistive Sensors with Low Detection Limits, Excellent Durability, and Multifunctional Sensing Capability. Sens. Actuators A Phys. 2023, 358, 114408. [Google Scholar] [CrossRef]
- Yuen, P.K.; Su, H.; Goral, V.N.; Fink, K.A. Three-Dimensional Interconnected Microporous Poly(Dimethylsiloxane) Microfluidic Devices. Lab Chip 2011, 11, 1541–1544. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(Dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, J.; Zhu, D.; Zheng, J.; Handschuh-Wang, S.; Zhou, X.; Zhang, J.; Liu, Y.; Liu, Z.; He, C.; et al. Hydrophilic Sponges for Leaf-Inspired Continuous Pumping of Liquids. Adv. Sci. 2017, 4, 1700028. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-Dimensional Nanonetworks for Giant Stretchability in Dielectrics and Conductors. Nat. Commun. 2012, 3, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.; Yang, K.; Wang, Z.; Chen, M.; Zhang, L.; Zhang, H.; Li, C. Fabrication of Highly Stretchable Conductors Based on 3D Printed Porous Poly(Dimethylsiloxane) and Conductive Carbon Nanotubes/Graphene Network. ACS Appl. Mater. Interfaces 2016, 8, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; He, Z.; Wang, P.; Yan, Y.; Ran, J. Hydrophobic Modified Activated Carbon Using PDMS for the Adsorption of VOCs in Humid Condition. Sep. Purif. Technol. 2020, 239, 116517. [Google Scholar] [CrossRef]
- Hickman, R.; Walker, E.; Chowdhury, S. TiO2-PDMS Composite Sponge for Adsorption and Solar Mediated Photodegradation of Dye Pollutants. J. Water Process Eng. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Zhang, W.; Fan, M.; Gong, Z.; Zhang, J. Graphene Oxide/Polydimethylsiloxane Composite Sponge for Removing Pb(Ii) from Water. RSC Adv. 2020, 10, 22492–22499. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Wang, W.; Bai, Z.; Zhang, Z.; Zhang, Y.; Zhang, S. The Fabrication of 3D Porous PDMS Sponge for Oil and Organic Solvent Absorption. Environ. Prog. Sustain. Energy 2019, 38, S86–S92. [Google Scholar] [CrossRef]
- Si, P.; Wang, J.; Zhao, C.; Xu, H.; Yang, K.; Wang, W. Preparation and Morphology Control of Three-Dimensional Interconnected Microporous PDMS for Oil Sorption. Polym. Adv. Technol. 2015, 26, 1091–1096. [Google Scholar] [CrossRef]
- Lamotte, M.; De Violet, P.; Garrigues, P.; Hardy, M. Evaluation of the Possibility of Detecting Benzenic Pollutants by Direct Spectrophotometry on PDMS Solid Absorbent. Anal. Bioanal. Chem. 2002, 372, 169–173. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Heo, J.H.; Jeon, S.; Park, J.H.; Kim, S.; Kang, H.W. Bio-Inspired Hollow PDMS Sponge for Enhanced Oil–Water Separation. J. Hazard. Mater. 2019, 365, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, C.; Cui, L.; Song, Z.; Zhao, X.; Ma, Y.; Jiang, L. Facile Preparation of the Porous PDMS Oil-Absorbent for Oil/Water Separation. Adv. Mater. Interfaces 2017, 4, 1600862. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Jovanovic, V.S.; Nikolic, J.; Ciric, S.; Milone, C.; Proverbio, E. Bentonite-PDMS Composite Foams for Oil Spill Recovery: Sorption Performance and Kinetics. J. Appl. Polym. Sci. 2022, 139, e53003. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable Superhydrophobic/Superoleophilic PDMS Sponges and Their Applications in Selective Oil Absorption and in Plugging Oil Leakages. J. Mater. Chem. A Mater. 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Tursi, A.; Chidichimo, F.; Bagetta, R.; Beneduci, A. BTX Removal from Open Aqueous Systems by Modified Cellulose Fibers and Evaluation of Competitive Evaporation Kinetics. Water 2020, 12, 3154. [Google Scholar] [CrossRef]
- Nagaraju, P.; Vijayakumar, Y.; Ramana Reddy, M.V. Room-Temperature BTEX Sensing Characterization of Nanostructured ZnO Thin Films. J. Asian Ceram. Soc. 2019, 7, 141–146. [Google Scholar] [CrossRef]
- Dean, B.J. Genetic toxicology of benzene, toluene, xylenes and phenols. Mutat. Res./Rev. Genet. Toxicol. 1978, 47, 75–97. [Google Scholar] [CrossRef]
- Wang, M.; Phillips, T.D. Green-Engineered Barrier Creams with Montmorillonite-Chlorophyll Clays as Adsorbents for Benzene, Toluene, and Xylene. Separations 2023, 10, 237. [Google Scholar] [CrossRef]
- Rinaldi, A.; Tamburrano, A.; Fortunato, M.; Sarto, M.S. A Flexible and Highly Sensitive Pressure Sensor Based on a PDMS Foam Coated with Graphene Nanoplatelets. Sensors 2016, 16, 2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent Progress in Fabrication and Application of Polydimethylsiloxane Sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Turco, A.; Pennetta, A.; Caroli, A.; Mazzotta, E.; Monteduro, A.G.; Primiceri, E.; de Benedetto, G.; Malitesta, C. Easy Fabrication of Mussel Inspired Coated Foam and Its Optimization for the Facile Removal of Copper from Aqueous Solutions. J. Colloid Interface Sci. 2019, 552, 401–411. [Google Scholar] [CrossRef]
- González-Rivera, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tinè, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 8, 616. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Jung, K.K.; Park, B.G.; Ko, J.S. Capacitive Oil Detector Using Hydrophobic and Oleophilic PDMS Sponge. Int. J. Precis. Eng. Manuf.-Green Technol. 2018, 5, 303–309. [Google Scholar] [CrossRef]
- Vadalà, M.; Kröll, E.; Küppers, M.; Lupascu, D.C.; Brunstermann, R. Hydrogen Production via Dark Fermentation by Bacteria Colonies on Porous PDMS-Scaffolds. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A Polydimethylsiloxane (PDMS) Sponge for the Selective Absorption of Oil from Water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhu, Y.; Zhao, G.; Tian, A.; Li, H.; Li, J.; Zhao, S.; Zhang, G.; Gao, A.; Cui, J.; et al. Preparation and Optimization of Conductive PDMS Composite Foams with Absorption-Dominated Electromagnetic Interference Shielding Performance via Silvered Aramid Microfibers. Eur. Polym. J. 2023, 191, 112029. [Google Scholar] [CrossRef]
- Sosnin, I.M.; Vlassov, S.; Akimov, E.G.; Agenkov, V.I.; Dorogin, L.M. Hydrophilic Polydimethylsiloxane-Based Sponges for Dewatering Applications. Mater. Lett. 2020, 263, 127278. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Jiang, X.; Li, D. Efficient Extraction Approach Based on Polydimethylsiloxane/ZIF-Derived Carbons Sponge Followed by GC–MS for the Determination of Volatile Compounds in Cumin. Food Chem. 2023, 405, 134775. [Google Scholar] [CrossRef]
- Turco, A.; Primiceri, E.; Frigione, M.; Maruccio, G.; Malitesta, C. An Innovative, Fast and Facile Soft-Template Approach for the Fabrication of Porous PDMS for Oil-Water Separation. J. Mater. Chem. A Mater. 2017, 5, 23785–23793. [Google Scholar] [CrossRef] [Green Version]
- Kovalenko, A.; Zimny, K.; Mascaro, B.; Brunet, T.; Mondain-Monval, O. Tailoring of the Porous Structure of Soft Emulsion-Templated Polymer Materials. Soft Matter 2016, 12, 5154–5163. [Google Scholar] [CrossRef]
- Timusk, M.; Nigol, I.A.; Vlassov, S.; Oras, S.; Kangur, T.; Linarts, A.; Šutka, A. Low-Density PDMS Foams by Controlled Destabilization of Thixotropic Emulsions. J. Colloid Interface Sci. 2022, 626, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Chen, P.; Du, W.; Feng, X.; Liu, B.F. Paraffin Oil Based Soft-Template Approach to Fabricate Reusable Porous PDMS Sponge for Effective Oil/Water Separation. Langmuir 2019, 35, 11123–11131. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Xia, N.; Yang, Z.; Zhang, C.; Pan, C.; Jin, D.; Zhang, J.; Majidi, C.; Zhang, L. Untethered small-scale magnetic soft robot with programmable magnetization and integrated multifunctional modules. Sci. Adv. 2022, 8, eabn8932. [Google Scholar] [CrossRef]
- Juchniewicz, M.; Stadnik, D.; Biesiada, K.; Olszyna, A.; Chudy, M.; Brzózka, Z.; Dybko, A. Porous Crosslinked PDMS-Microchannels Coatings. Sens. Actuators B Chem. 2007, 126, 68–72. [Google Scholar] [CrossRef]
- Tebboth, M.; Jiang, Q.; Kogelbauer, A.; Bismarck, A. Inflatable Elastomeric Macroporous Polymers Synthesized from Medium Internal Phase Emulsion Templates. ACS Appl. Mater. Interfaces 2015, 7, 19243–19250. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Yuan, D.; Xu, S.; Wang, L. Gas Foaming Guided Fabrication of 3D Porous Plasmonic Nanoplatform with Broadband Absorption, Tunable Shape, Excellent Stability, and High Photothermal Efficiency for Solar Water Purification. Adv. Funct. Mater. 2020, 30, 2003995. [Google Scholar] [CrossRef]
- Chen, S.; Zhuo, B.; Guo, X. Large Area One-Step Facile Processing of Microstructured Elastomeric Dielectric Film for High Sensitivity and Durable Sensing over Wide Pressure Range. ACS Appl. Mater. Interfaces 2016, 8, 20364–20370. [Google Scholar] [CrossRef]
- Pasquali, I.; Bettini, R.; Giordano, F. Thermal Behaviour of Diclofenac, Diclofenac Sodium and Sodium Bicarbonate Compositions. J Therm Anal Calorim. 2007, 90, 903–907. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of Surface Roughness on Contact Angle Hysteresis and Spreading Work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Nakajima, A.; Abe, K.; Hashimoto, K.; Watanabe, T. Preparation of Hard Super-Hydrophobic Films with Visible Light Transmission. Thin Solid Films. 2000, 376, 140–143. [Google Scholar] [CrossRef]
- Labouriau, A.; Cox, J.D.; Schoonover, J.R.; Patterson, B.M.; Havrilla, G.J.; Stephens, T.; Taylor, D. Mössbauer, NMR and ATR-FTIR Spectroscopic Investigation of Degradation in RTV Siloxane Foams. Polym. Degrad. Stab. 2007, 92, 414–424. [Google Scholar] [CrossRef]
- Ren, J.; Wu, F.; Shang, E.; Li, D.; Liu, Y. 3D Printed Smart Elastomeric Foam with Force Sensing and Its Integration with Robotic Gripper. Sens. Actuators A Phys. 2023, 349, 113998. [Google Scholar] [CrossRef]
- Zhai, W.; Xia, Q.; Zhou, K.; Yue, X.; Ren, M.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Multifunctional Flexible Carbon Black/Polydimethylsiloxane Piezoresistive Sensor with Ultrahigh Linear Range, Excellent Durability and Oil/Water Separation Capability. Chem. Eng. J. 2019, 372, 373–382. [Google Scholar] [CrossRef]
- Boscaini, E.; Alexander, M.L.; Prazeller, P.; Märk, T.D. Investigation of Fundamental Physical Properties of a Polydimethylsiloxane (PDMS) Membrane Using a Proton Transfer Reaction Mass Spectrometer (PTRMS). Int. J. Mass Spectrom. 2004, 239, 179–186. [Google Scholar] [CrossRef]
- Zepeda, A.; Texier, A.C.; Razo-Flores, E.; Gomez, J. Kinetic and Metabolic Study of Benzene, Toluene and m-Xylene in Nitrifying Batch Cultures. Water Res. 2006, 40, 1643–1649. [Google Scholar] [CrossRef]
- Qian, C.; Guo, Q.; Xu, M.; Yuan, Y.; Yao, J. Improving the SERS Detection Sensitivity of Aromatic Molecules by a PDMS-Coated Au Nanoparticle Monolayer Film. RSC Adv. 2015, 5, 53306–53312. [Google Scholar] [CrossRef]
- Calabrese, L.; Piperopoulos, E.; Jovanovic, V.S.; Mitic, V.; Mitic, M.; Milone, C.; Proverbio, E. Oil Spill Remediation: Selectivity, Sorption, and Squeezing Capacity of Silicone Composite Foams Filled with Clinoptilolite. J. Appl. Polym. Sci. 2022, 139, e52637. [Google Scholar] [CrossRef]
- Sun, X.; Shi, K.; Mo, S.; Mei, J.; Rong, J.; Wang, S.; Zheng, X.; Li, Z. A Sustainable Reinforced-Concrete-Structured Sponge for Highly-Recyclable Oil Adsorption. Sep. Purif. Technol. 2023, 305, 122483. [Google Scholar] [CrossRef]
- Gupta, R.; Kulkarni, G.U. Removal of Organic Compounds from Water by Using a Gold Nanoparticle-Poly(Dimethylsiloxane) Nanocomposite Foam. ChemSusChem 2011, 4, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Bindra, H.S.; Jain, S.; Nayak, R. Sustainable Lotus Leaf Wax Nanocuticles Integrated Polydimethylsiloxane Sorbent for Instant Removal of Oily Waste from Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127937. [Google Scholar] [CrossRef]
- Scott, A.; Gupta, R.; Kulkarni, G.U. A Simple Water-Based Synthesis of Au Nanoparticle/PDMS Composites for Water Purification and Targeted Drug Release. Macromol. Chem. Phys. 2010, 211, 1640–1647. [Google Scholar] [CrossRef]
- Mo, S.; Mei, J.; Liang, Q.; Li, Z. Repeatable Oil-Water Separation with a Highly-Elastic and Tough Amino-Terminated Polydimethylsiloxane-Based Sponge Synthesized Using a Self-Foaming Method. Chemosphere 2021, 271, 129827. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(Dimethylsiloxane) Oil Absorbent with a Three-Dimensionally Interconnected Porous Structure and Swellable Skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef]
- Qiu, S.; Bi, H.; Hu, X.; Wu, M.; Li, Y.; Sun, L. Moldable Clay-like Unit for Synthesis of Highly Elastic Polydimethylsiloxane Sponge with Nanofiller Modification. RSC Adv. 2017, 7, 10479–10486. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.N.H.; Kabiri, S.; Sim, T.R.; Losic, D. Selective Adsorption of Oil-Water Mixtures Using Polydimethylsiloxane (PDMS)-Graphene Sponges. Environ. Sci. 2015, 1, 298–305. [Google Scholar] [CrossRef]
- Guo, Z.; Long, B.; Gao, S.; Luo, J.; Wang, L.; Huang, X.; Wang, D.; Xue, H.; Gao, J. Carbon Nanofiber Based Superhydrophobic Foam Composite for High Performance Oil/Water Separation. J. Hazard. Mater. 2021, 402, 123838. [Google Scholar] [CrossRef]










| PDMS Foam | NaHCO3 (%) | Acetic Acid (%) | Curing Temperature (°C) |
|---|---|---|---|
| 1:1(100) | 5% | 5% | 100 |
| 1:2(100) | 5% | 10% | 100 |
| 2:1(100) | 10% | 5% | 100 |
| 1:2(80) | 5% | 10% | 80 |
| 1:2(120) | 5% | 10% | 120 |
| PDMS Foam | Density (g/cm3) | Porosity % | Water Contact Angle |
|---|---|---|---|
| 1:1(100) | 0.216 | 79 | 119.33 |
| 1:2(100) | 0.247 | 76 | 139.42 |
| 2:1(100) | 0.279 | 73 | 128.17 |
| 1:2(80) | 0.348 | 66 | 110.42 |
| 1:2(120) | 0.126 | 88 | 131.42 |
| Wavenumber (cm−1) | Assignment |
|---|---|
| 786.71 | Si-CH3 stretching |
| 843.75 | -CH3 rock |
| 1008.29 | Si-O-Si stretching |
| 1257.57 | C-H bending |
| 1411.58 | C-H bending |
| 2962.10 | C-H stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatawi, L.; Abdullah, A.H.; Jamil, S.N.A.M.; Yunus, R. A Facile and Green Synthesis of Hydrophobic Polydimethylsiloxane Foam for Benzene, Toluene, and Xylene Removal. Separations 2023, 10, 377. https://doi.org/10.3390/separations10070377
Alatawi L, Abdullah AH, Jamil SNAM, Yunus R. A Facile and Green Synthesis of Hydrophobic Polydimethylsiloxane Foam for Benzene, Toluene, and Xylene Removal. Separations. 2023; 10(7):377. https://doi.org/10.3390/separations10070377
Chicago/Turabian StyleAlatawi, Lila, Abdul Halim Abdullah, Siti Nurul Ain Md. Jamil, and Robiah Yunus. 2023. "A Facile and Green Synthesis of Hydrophobic Polydimethylsiloxane Foam for Benzene, Toluene, and Xylene Removal" Separations 10, no. 7: 377. https://doi.org/10.3390/separations10070377