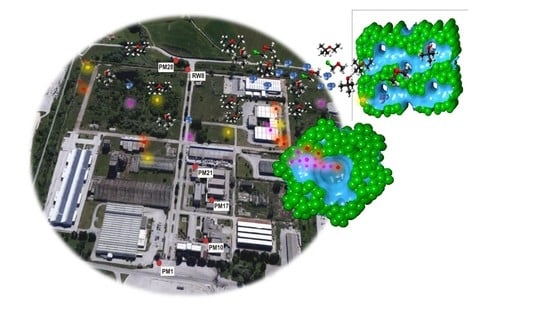
Evaluation for the Removal Efficiency of VOCs and Heavy Metals by Zeolites-Based Materials in the Wastewater: A Case Study in the Tito Scalo Industrial Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Materials
2.3. Adsorption Experiments
2.4. Inductively Coupled Plasma Optical Emission Spectrometry
2.5. Gas Chromatography
2.6. Quality Assurance
2.7. Thermal Analyses
2.8. X-ray Powder Diffraction Data Collection and Refinement Strategy
3. Results and Discussion
3.1. Adsorption
3.2. Structural and Thermal Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: A critical review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef] [PubMed]
- CEC. Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). 2006. Available online: https://www.desitek.dk/sites/default/files/media/files/DEHN-REACH-Certificate.pdf (accessed on 20 November 2020).
- Reemtsma, T.; Berger, U.; Arp, H.P.H.; Gallard, H.; Knepper, T.P.; Neumann, M.; Quintana, J.B.; Voogt, P.D. Mind the Gap: Persistent and Mobile Organic Compounds—Water Contaminants That Slip Through. Environ. Sci. Technol. 2016, 50, 10308–10315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darko, G.; Akoto, O.; Oppong, C. Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana. Chemosphere 2008, 72, 21–24. [Google Scholar] [CrossRef] [PubMed]
- dela Cruz, A.L.N.; Cook, R.L.; Dellinger, B.; Lomnicki, S.M.; Donnelly, K.C.; Kelley, M.A.; Cosgriff, D. Assessment of environmentally persistent free radicals in soils and sediments from three Superfund sites. Environ. Sci. Process. Impacts 2014, 16, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISPRA, S.I.N. Siti di Interesse Nazionale—Stato Delle Procedure Per la Bonifica. Dicembre 2018. Available online: http://bit.ly/2MjNp9P (accessed on 20 November 2020).
- Perego, C.; Bagatin, R.; Tagliabue, M.; Vignola, R. Zeolites and related mesoporous materials for multi-talented environmental solutions. Microporous Mesoporous Mater. 2013, 166, 37–49. [Google Scholar] [CrossRef]
- Maretto, M.; Blanchi, F.; Vignola, R.; Canepari, S.; Baric, M.; Iazzoni, R.; Tagliabue, M.; Papini, M.P. Microporous and mesoporous materials for the treatment of wastewater produced by petrochemical activities. J. Clean. Prod. 2014, 77, 22–34. [Google Scholar] [CrossRef]
- Colella, C.; Čejka, J.; van Bekkum, H.; Corma, A.; Schueth, F. (Eds.) Introduction to Zeolite Science and Practice, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 999–1035. [Google Scholar]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Vignola, R.; Bagatin, R.; Alessandra De Folly, D.; Flego, C.; Nalli, M.; Ghisletti, D.; Millini, R.; Sisto, R. Zeolites in a permeable reactive barrier (PRB): One year of field experience in a refinery groundwater—Part 1: The performances. Chem. Eng. J. 2011, 178, 204–209. [Google Scholar] [CrossRef]
- Vignola, R.; Bagatin, R.; Alessandra De Folly, D.; Massara, E.P.; Ghisletti, D.; Millini, R.; Sisto, R. Zeolites in a permeable reactive barrier (PRB): One-year of field experience in a refinery groundwater. Part 2: Zeolite characterization. Chem. Eng. J. 2011, 178, 210–216. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Zhang, Y.; Zhang, C.; Li, X.; Chen, Z.; Huang, J.; Li, X.; Flores, G.; Kamon, M. Column test-based optimization of the permeable reactive barrier (PRB) technique for remediating groundwater contaminated by landfill leachates. J. Contam. Hydrol. 2014, 168, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Northcott, K.A.; Bacus, J.; Taya, N.; Komatsu, Y.; Perera, J.M.; Stevens, G.W. Synthesis and characterization of hydrophobic zeolite for the treatment of hydrocarbon contaminated ground water. J. Hazard. Mater. 2010, 183, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhou, Y.; Peng, H.; Huang, S.; Qin, P.; Zhang, J.; Yang, Y.; Luo, L.; Zhang, X. Current progress in remediation of chlorinated volatile organic compounds: A review. J. Ind. Eng. Chem. 2018, 62, 106–119. [Google Scholar] [CrossRef]
- Syed, F.H.; Egleston, C.; Datta, R. Tert-Amyl methyl ether (TAME). Thermodynamic analysis of reaction equilibria in the liquid phase. J. Chem. Eng. Data 2000, 45, 319–323. [Google Scholar] [CrossRef]
- Colombo, F.; Cori, L.; Dallora, L.; Delogu, P. Equilibrium Constant for the Methyl tert-Butyl Ether Liquid-Phase Synthesis by use of UNIFAC. Ind. Eng. Chem. Fundam. 1983, 22, 219–223. [Google Scholar] [CrossRef]
- Safronov, V.V.; Sharanov, K.G.; Rozhnov, A.M.; Alenin, V.J.; Sidorov, S.A. Thermodynamics of Sythesis of tert-Amyl Methyl Ether. Zh. Prikl. Khim.(Leningrad) 1989, 62, 824–828. [Google Scholar]
- Rehfinger, A.; Hoffmann, U. Kinetics of Methyl Tertiary Butyl Ether Liquid Phase Synthesis Catalyzed by Ion Exchange Resin-I. Intrinsic Rate Expression in Liquid-Phase Activities. Chem. Eng. Sci. 1990, 45, 1605–1617. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Cunill, F.; Vila, M.; Tejero, J.; Iborra, M. Equilibrium Constants for Methyl tert-Butyl Ether Liquid Phase Synthesis. J. Chem. Eng. Data 1992, 37, 339–343. [Google Scholar] [CrossRef]
- Vila, M.; Cunill, F.; Izquierdo, J.F.; Tejero, J.; Iborra, M. Equilibrium Constants for Ethyl tert-Butyl Ether Liquid-Phase Synthesis. Chem. Eng. Commun. 1993, 124, 223–232. [Google Scholar] [CrossRef]
- Jensen, K.L.; Datta, R. Ethers from Ethanol. 1. Equilibrium Thermodynamic Analysis of the Liquid-Phase Ethyl tert-Butyl Ether Reaction. Ind. Eng. Chem. Res. 1995, 34, 392–399. [Google Scholar] [CrossRef]
- Zhang, T.; Datta, R. Integral Analysis of Methyl tert-Butyl Ether Synthesis Kinetics. Ind. Eng. Chem. Res. 1995, 34, 730–740. [Google Scholar] [CrossRef]
- Kitchaiya, P.; Datta, R. Ethers from Ethanol. 2. Reaction Equilibria of Simultaneous tert-Amyl Ethyl Ether Synthesis and Isoamylene Isomerization. Ind. Eng. Chem. Res. 1995, 34, 1092–1101. [Google Scholar] [CrossRef]
- Zhang, T.; Datta, R. Ethers from Ethanol. 3. Equilibrium Conversion and Selectivity Limitations in the Liquid-Phase Synthesis of Two tert-Hexyl Ethyl Ethers. Ind. Eng. Chem. Res. 1995, 34, 2237–2246. [Google Scholar] [CrossRef]
- Atik, Z.; Lourddani, K. Densities and Volumetric Properties of Binary and Ternary Mixtures of Diisopropyl Ether, Fluorobenzene, α, α, α-Trifluorotoluene, and Ethanol at Temperature 298.15 K and Pressure 101 kPa. J. Solut. Chem. 2006, 35, 1453–1466. [Google Scholar] [CrossRef]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Guzzinati, R.; Sarti, E.; Catani, M.; Costa, V.; Pagnoni, A.; Martucci, A.; Rodeghero, E.; Capitani, D.; Pietrantonio, M.; Cavazzini APasti, L. Formation of Supramolecular Clusters at the Interface of Zeolite X Following the Adsorption of Rare-Earth Cations and Their Impact on the Macroscopic Properties of the Zeolite. Chem. Phys. Chem. 2018, 19, 2208–2217. [Google Scholar] [CrossRef]
- El Brihi, T.; Jaubert, J.N.; Barth, D.; Perrin, L. Determining volatile organic compounds’ adsorption isotherms on dealuminated Y zeolite and correlation with different models. J. Chem. Eng. Data 2002, 47, 1553–1557. [Google Scholar] [CrossRef]
- Monneyron, P.; Manero, M.H.; Foussard, J.N. Measurement and modeling of single-and multi-component adsorption equilibria of VOC on high-silica zeolites. Environ. Sci. Technol. 2003, 37, 2410–2414. [Google Scholar] [CrossRef] [Green Version]
- Benmaamar, Z.; Bengueddach, A. Correlation with different models for adsorption isotherms of m-xylene and toluene on zeolites. J. Appl. Sci. Environ. Sanit. 2007, 2, 43–56. [Google Scholar]
- Rodeghero, E.; Chenet, T.; Martucci, A.; Ardit, M.; Sarti, E.; Pasti, L. Selective adsorption of toluene and n-hexane binary mixture from aqueous solution on zeolite ZSM-5: Evaluation of competitive behavior between aliphatic and aromatic compounds. Catal. Today 2020, 345, 157–164. [Google Scholar] [CrossRef]
- Martucci, A.; Leardini, L.; Nassi, M.; Sarti, E.; Bagatin, R.; Pasti, L. Removal of emerging organic contaminants from aqueous systems: Adsorption and location of methyl-tertiary-butylether on synthetic ferrierite. Mineral. Mag. 2014, 78, 1161–1175. [Google Scholar] [CrossRef]
- Pasti, L.; Rodeghero, E.; Sarti, E.; Bosi, V.; Cavazzini, A.; Bagatin, R.; Martucci, A. Competitive adsorption of VOCs from binary aqueous mixtures on zeolite ZSM-5. RSC Adv. 2016, 6, 54544–54552. [Google Scholar] [CrossRef]
- Martucci, A.; Braschi, I.; Bisio, C.; Sarti, E.; Rodeghero, E.; Bagatin, R.; Pasti, L. Influence of water on the retention of methyl tertiary-butyl ether by high silica ZSM-5 and Y zeolites: A multidisciplinary study on the adsorption from liquid and gas phase. RSC Adv. 2015, 5, 86997–87006. [Google Scholar] [CrossRef]
- ISPRA. Measurement Procedure for the Determination of Total Hydrocarbons in Waters, Handbooks and Guidelines 23/2015. Available online: https://www.isprambiente.gov.it/en/publications/publications-of-the-agency-sistem/measurement-procedure-for-the-determination-of-total-hydrocarbons-in-waters (accessed on 20 November 2020).
- R. US-EPA. Definition and Procedure for the Determination of the Method Detection Limit, 40 CFR Ch. I, 7–1–03 Ed. 2003. Available online: https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf (accessed on 20 November 2020).
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748. 1994. Available online: https://www.ncnr.nist.gov/xtal/software/gsas.html (accessed on 20 November 2020).
- Toby, B.H. Expgui, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated Solvents in Groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Pasti, L.; Rodeghero, E.; Beltrami, G.; Ardit, M.; Sarti, E.; Chenet, T.; Stevanin, C.; Martucci, A. Insights into Adsorption of Chlorobenzene in High Silica MFI and FAU Zeolites Gained from Chromatographic and Diffractometric Techniques. Minerals 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Pasti, L.; Martucci, A.; Nassi, M.; Cavazzini, A.; Alberti, A.; Bagatin, R. The role of water in DCE adsorption from aqueous solutions onto hydrophobic zeolites. Microporous Mesoporous Mater. 2012, 160, 182–193. [Google Scholar] [CrossRef]
- Rodeghero, E.; Martucci, A.; Cruciani, G.; Bagatin, R.; Sarti, E.; Bosi, V.; Pasti, L. Kinetics and dynamic behaviour of toluene desorption from ZSM-5 using in situ high-temperature synchrotron powder X-ray diffraction and chromatographic techniques. Catal. Today 2016, 277, 118–125. [Google Scholar] [CrossRef]
- Rodeghero, E.; Pasti, L.; Sarti, E.; Cruciani, G.; Bagatin, R.; Martucci, A. Temperature-induced desorption of methyl tert-butyl ether confined on ZSM-5: An in situ synchrotron XRD powder diffraction study. Minerals 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, N.; Oshiro, T.; Tan, S.; Nishi, K.; Yokomori, Y. Adsorption process of phenol on silicalite-1 and crystal structure of phenol8. 0–silicalite-1 using a single crystal X-ray diffraction method. Microporous Mesoporous Mater. 2013, 169, 168–175. [Google Scholar] [CrossRef]
- Martucci, A.; Rodeghero, E.; Pasti, L.; Bosi, V.; Cruciani, G. Adsorption of 1, 2-dichloroethane on ZSM-5 and desorption dynamics by in situ synchrotron powder X-ray diffraction. Microporous Mesoporous Mater. 2015, 215, 175–182. [Google Scholar] [CrossRef]
- Martucci, A.; Pasti, L.; Nassi, M.; Alberti, A.; Arletti, R.; Bagatin, R.; Vignola, R.; Sticca, R. Adsorption mechanism of 1, 2-dichloroethane into an organophilic zeolite mordenite: A combined diffractometric and gas chromatographic study. Microporous Mesoporous Mater. 2012, 151, 358–367. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry—An Introduction Emphasizing Chemical Equilibria in Natural Waters; Wiley Sons: Hoboken, NJ, USA, 1981. [Google Scholar]
- Trigueiro, F.E.; Monteiro, D.F.J.; Zotin, F.M.Z.; Sousa-Aguiar, E.F. Thermal stability of Y zeolites containing different rare earth cations. J. Alloy. Compd. 2002, 344, 337–341. [Google Scholar] [CrossRef]
- Gal, I.J.; Radovanov, P. Ion-exchange equilibria of synthetic 13X zeolite with Ni2+, Co2+, Zn2+ and Cd2+ ions. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1975, 71, 1671–1677. [Google Scholar] [CrossRef]
- Esposito, S.; Ferone, C.; Pansini, M.; Bonaccorsi, L.; Proverbio, E. A comparative study of the thermal transformations of Ba-exchanged zeolites A, X and LSX. J. Eur. Ceram. Soc. 2004, 24, 2689–2697. [Google Scholar] [CrossRef]
- Khaleghian-Moghadam, R.; Seyedeyn-Azad, F. A study on the thermal behavior of low silica X-type zeolite ion-exchanged with alkaline earth cations. Microporous Mesoporous Mater. 2009, 120, 285–293. [Google Scholar] [CrossRef]
- Babé, A.; Labbé, D.; Monot, F.; Greer, C.W.; Fayolle-Guichard, F. Biodegradability of oxygenates by microflora from MTBE-contaminated sites: New molecular tools. HDB Environ. Chem. 2007, 5, 75–98. [Google Scholar]
- Painter, B.D.; Milke, M.W. Comparison of factorial and scenario analysis methods for assessing uncertainty in the design of permeable reactive barriers. Groundwater Monit. Remediat. 2007, 27, 102–110. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Careghini, A.; Saponaro, S.; Sezenna, E.; Daghio, M.; Franzetti, A.; Gandolfi, I.; Bestetti, G. Lab-scale tests and numerical simulations for in situ treatment of polluted groundwater. J. Hazard. Mater. 2015, 287, 162–170. [Google Scholar] [CrossRef]
- Sharma, H.D.; Reddy, K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies; Wiley Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Medvidović, N.V.; Nuić, I.; Ugrina, M.; Trgo, M. Evaluation of natural zeolite as a material for permeable reactive barrier for remediation of zinc-contaminated groundwater based on column study. Water Air Soil Pollut. 2018, 229, 367. [Google Scholar] [CrossRef]
- Woinarski, A.Z.; Stevens, G.W.; Snape, I. A natural zeolite permeable reactive barrier to treat heavy-metal contaminated waters in Antarctica: Kinetic and fixed-bed studies. Process. Saf. Environ. Prot. 2006, 84, 109–116. [Google Scholar] [CrossRef]
- Vignola, R.; Cova, U.; Fabiani, F.; Grillo, G.; Molinari, M.; Sbardellati, R.; Sisto, R. Remediation of hydrocarbon contaminants in groundwater using specific zeolites in full-scale pumptreat and demonstrative permeable barrier tests. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 573–576. [Google Scholar]
- Vaezihir, A.; Bayanlou, M.B.; Ahmadnezhad, Z.; Barzegari, G. Remediation of BTEX plume in a continuous flow model using zeolite-PRB. J. Contam. Hydrol. 2020, 230, 103604. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Birke, V. Permeable Reactive Barrier: Sustainable Groundwater Remediation; Taylor Francis: Oxfordshire, UK, 2015; p. 333. [Google Scholar]
- Asante-Duah, K. Management of Contaminated Site Problems; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Ye, J.; Chen, X.; Chen, C.; Bate, B. Emerging sustainable technologies for remediation of soils and groundwater in a municipal solid waste landfill site—A review. Chemosphere 2019, 227, 681–702. [Google Scholar] [CrossRef]
- Chandra, S.; Chauhan, L.K.S.; Murthy, R.C.; Saxena, P.N.; Pande, P.N.; Gupta, S.K. Comparative biomonitoring of leachates from hazardous solid waste of two industries using Allium test. Sci. Total Environ. 2005, 347, 46–52. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Song, Y.; Sarmah, A.K.; Li, X.; Tack, F.M. Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: A review. J. Control. Release 2018, 283, 200–213. [Google Scholar] [CrossRef]
- Johnson, G.R.; Zhang, Z.; Brusseau, M.L. Characterizing and quantifying the impact of immiscible-liquid dissolution and nonlinear, rate-limited sorption/desorption on low-concentration elution tailing. Water Resour. Res. 2003, 39, 39. [Google Scholar] [CrossRef]
- Chapman, S.W.; Parker, B.L. Plume persistence due to aquitard back diffusion following dense nonaqueous phase liquid source removal or isolation. Water Resour. Res. 2005, 41, 12. [Google Scholar] [CrossRef]
- Krembs, F.J.; Siegrist, R.L.; Crimi, M.L.; Furrer, R.F.; Petri, B.G. ISCO for groundwater remediation: Analysis of field applications and performance. Groundwater Monit. Remediat. 2010, 30, 42–53. [Google Scholar] [CrossRef]
- Painter, B.D. Optimisation of Permeable Reactive Barrier Systems for the Remediation of Contaminated Groundwater. Ph.D. Thesis, Lincoln University, Jackson, MO, USA, 2005. [Google Scholar]
- Hadley, P.W.; Newell, C.J. Groundwater remediation: The next 30 years. Groundwater 2012, 50, 669–678. [Google Scholar] [CrossRef]
- Stroo, H.F.; Unger, M.; Ward, C.H.; Kavanaugh, M.C.; Vogel, C.; Leeson, A.; Marqusee, J.; Smith, B.P. Peer reviewed: Remediating chlorinated solvent source zones. Environ. Sci. Technol. 2003, 37, 224A–230A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Gao, Y.; Zhang, Y.; Dong, W.; Lai, M. Remediation of lead and cadmium from simulated groundwater in loess region in northwestern China using permeable reactive barrier filled with environmentally friendly mixed adsorbents. Environ. Sci. Pollut. Res. 2018, 25, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.; Abd Ali, Z.T. Groundwater protection from lead contamination using granular dead anaerobic sludge biosorbent as permeable reactive barrier. Desalin. Water Treat. 2016, 57, 3891–3903. [Google Scholar] [CrossRef]




| Selected Zeolites | Framework Topology |
|---|---|
| 13X: Na 90.04 [Al 92.64 Si 99.78 O 384] • 200.03 H2O Cation type: Na Si/Al (mol/mol): 1.08 Channel dimensionality: Topological (pore opening 6-ring): 3-dimensional SSABET (m2 g−1): 791; SSAmicrop (m2 g−1): 731; Vp (cm3 g−1): 0.301; Vmicrop (cm3 g−1): 0.267 | [FAU] |
| ZSM-5: [Si96O192] Cation type: NH4 Si/Al (mol/mol): 500 Channel dimensionality: Topological (pore opening 6-ring): 3-dimensional SSABET (m2 g−1): 550; SSAmicrop (m2 g−1): 355; Vp (cm3 g−1): 0.52; Vmicrop (cm3 g−1): 0.09 | [MFI] |
| 13X | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 | |
|---|---|---|---|---|---|---|---|
| Space group | Fd-3m | Fd-3m | Fd-3m | Fd-3m | Fd-3m | Fd-3m | Fd-3m |
| a = b = c (Å) | 24.9859(3) | 24.8980(3) | 24.8762(2) | 24.9160(3) | 24.9067(2) | 24.8780(3) | 24.8773(3) |
| α = β = γ (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| V (Å3) | 15,598.70(3) | 15,434.52(3) | 15,394.00(7) | 15,467.9(3) | 15,450.68(2) | 15,397.39(3) | 15,396.00(6) |
| Wavelength (Å): Cu Kα1 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 |
| Cu Kα2 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 |
| Refined 2θ (°) range | 10–120° | 10–120° | 10–120° | 10–120° | 10–120° | 10–120° | 10–120° |
| ZSM-5 | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 | |
|---|---|---|---|---|---|---|---|
| Space group | P21/n | P21/n | P21/n | P21/n | P21/n | P21/n | P21/n |
| a (Å) | 19.8999(5) | 19.9018(4) | 19.8936(4) | 19.8879(9) | 19.8934(6) | 19.8946(5) | 19.8968(5) |
| b (Å) | 20.1174(6) | 20.1256(4) | 20.1166(4) | 20.1107(7) | 20.1152(4) | 20.1176(4) | 20.1216(8) |
| c (Å) | 13.3892(4) | 13.3877(3) | 13.3817(3) | 13.3834(4) | 13.3834(4) | 13.3840(4) | 13.3834(8) |
| α (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| β (°) | 90.546(3) | 90.558(2) | 90.567(2) | 90.550(4) | 90.555(4) | 90.557(5) | 90.557(5) |
| γ (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| V (Å3) | 5359.90(3) | 5362.00(2) | 5355.00(2) | 5352.60(4) | 5355.23(3) | 5356.42(2) | 5357.80(1 |
| Wavelength (Å): Cu Kα1 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 | 1.540593 |
| Cu Kα2 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 | 1.544427 |
| Refined 2θ (°) range | 10°–120° | 10°–120° | 10°–120° | 10°–120° | 10°–120° | 10°–120° | 10°–120° |
| VOCs | |||
| Sample | C0 (mg L−1) | q (mg g−1) | Removal Efficiency (%) |
| PM1 | 164 ± 12.63 | 37 ± 1.30 | 99.65 |
| PM10 | 2.6 ± 0.26 | 0.6 ± 0.20 | 96.89 |
| PM17 | 20 ± 1.77 | 4.3 ± 0.51 | 98.54 |
| PM21 | 5.5 ± 0.95 | 1.2 ± 0.45 | 97.58 |
| PM28 | 0.75 ± 0.09 | 0.2 ± 0.11 | 87.31 |
| RW8 | 3.4 ± 0.28 | 0.8 ± 0.21 | 96.79 |
| Fe | |||
| Sample | C0 (µg L−1) | q (µg g−1) | Removal Efficiency (%) |
| PM1 | - | - | - |
| PM10 | 56.8 ± 0.56 | 29.4 ± 0.47 | 53.27 |
| PM17 | 2.8 ± 0.24 | 2.8 ± 0.35 | 100 |
| PM21 | 1.5 ± 0.11 | 1.5 ± 0.31 | 100 |
| PM28 | 4.9 ± 0.18 | 4.8 ± 0.35 | 100 |
| RW8 | 6.6 ± 0.58 | 6.5 ± 0.38 | 100 |
| Mn | |||
| Sample | C0 (µg L−1) | q (µg g−1) | Removal Efficiency (%) |
| PM1 | 962 ± 5.2 | 694 ± 1.91 | 73.00 |
| PM10 | 1104 ± 7.2 | 1014 ± 3.80 | 94.35 |
| PM17 | 578.5 ± 0.90 | 554 ± 1.26 | 98.40 |
| PM21 | 414 ± 1.00 | 412 ± 1.23 | 99.83 |
| PM28 | - | - | - |
| RW8 | 1745 ± 4.3 | 1172 ± 3.97 | 67.99 |
| 13X | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 | |
|---|---|---|---|---|---|---|---|
| CFA (Å2) | 43.29 | 43.23 | 43.43 | 43.45 | 43.76 | 43.28 | 47.29 |
| ε | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.00 | 1.04 |
| Straight channel (SC) [010] Ring A | ZSM-5 | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 |
| CFA (Å2) | 22.69 | 21.05 | 23.67 | 24.31 | 23.82 | 23.83 | 24.03 |
| ε | 1.03 | 1.08 | 1.06 | 1.11 | 1.05 | 1.05 | 1.09 |
| Straight channel (SC) [010] Ring B | ZSM-5 | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 |
| CFA (Å2) | 23.03 | 22.42 | 23.08 | 23.50 | 23.01 | 23.01 | 23.61 |
| ε | 1.02 | 1.08 | 1.03 | 1.10 | 1.04 | 1.04 | 1.06 |
| Sinusoidal or zigzag channel (ZZ) [100] Ring A | ZSM-5 | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 |
| CFA (Å2) | 21.65 | 21.59 | 23.54 | 23.98 | 23.63 | 23.63 | 24.07 |
| ε | 1.05 | 1.08 | 1.08 | 1.12 | 1.08 | 1.08 | 1.06 |
| Sinusoidal or zigzag channel (ZZ) [100] Ring B | ZSM-5 | PM1 | PM10 | PM17 | PM21 | PM28 | RW8 |
| CFA (Å2) | 22.65 | 21.49 | 22.70 | 24.27 | 22.87 | 22.87 | 23.05 |
| ε | 1.06 | 1.12 | 1.09 | 1.10 | 1.10 | 1.10 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancinelli, M.; Arfè, A.; Martucci, A.; Pasti, L.; Chenet, T.; Sarti, E.; Vergine, G.; Belviso, C. Evaluation for the Removal Efficiency of VOCs and Heavy Metals by Zeolites-Based Materials in the Wastewater: A Case Study in the Tito Scalo Industrial Area. Processes 2020, 8, 1519. https://doi.org/10.3390/pr8111519
Mancinelli M, Arfè A, Martucci A, Pasti L, Chenet T, Sarti E, Vergine G, Belviso C. Evaluation for the Removal Efficiency of VOCs and Heavy Metals by Zeolites-Based Materials in the Wastewater: A Case Study in the Tito Scalo Industrial Area. Processes. 2020; 8(11):1519. https://doi.org/10.3390/pr8111519
Chicago/Turabian StyleMancinelli, Maura, Antonella Arfè, Annalisa Martucci, Luisa Pasti, Tatiana Chenet, Elena Sarti, Giulia Vergine, and Claudia Belviso. 2020. "Evaluation for the Removal Efficiency of VOCs and Heavy Metals by Zeolites-Based Materials in the Wastewater: A Case Study in the Tito Scalo Industrial Area" Processes 8, no. 11: 1519. https://doi.org/10.3390/pr8111519