Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth
Abstract
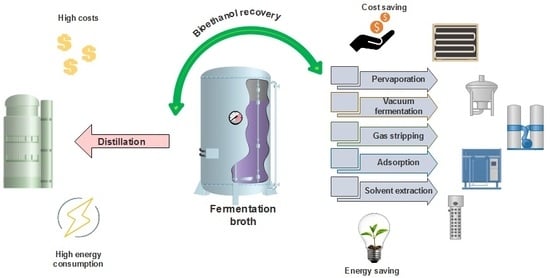
:1. Introduction
2. Alternative Ethanol Recovery Techniques
2.1. Pervaporation
2.2. Gas Stripping
2.3. Vacuum Fermentation
2.4. Adsorption
2.5. Solvent Extraction
3. Economic Comparison Between the Different Ethanol Recovery Techniques
4. Conclusions and Future Trends
Author Contributions
Acknowledgments
Conflicts of Interest
References
- 2014 Energy and Climate Outlook. Available online: https://globalchange.mit.edu/sites/default/files/newsletters/files/2014%20Energy%20&%20Climate%20Outlook.pdf (accessed on 3 July 2019).
- Dudley, B. BP Statistical Review of World Energy-All data, 1965-2018. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 3 July 2019).
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; Praeger: Santa Barbara, CA, USA, 2016. [Google Scholar]
- Chen, W.T.; Shu, C.M. CO2 reduction for a low-carbon community: A city perspective in Taiwan. Sep. Purif. Technol. 2012, 94, 154–159. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian biomass for alternative renewable biofuel. Renew. energy. 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Vázquez-Ojeda, M.; Segovia-Hernández, J.G.; Hernández, S.; Hernández-Aguirre, A.; Kiss, A.A. Design and optimization of an ethanol dehydration process using stochastic methods. Sep. Purif. Technol. 2013, 105, 90–97. [Google Scholar] [CrossRef]
- Delgado, J.; Águeda, V.; Uguina, M.; Sotelo, J.; García-Sanz, A.; García, A. Separation of ethanol-water liquid mixtures by adsorption on BPL activated carbon with air regeneration. Sep. Purif. Technol. 2015, 149, 370–380. [Google Scholar] [CrossRef]
- Lichts, F.O. World Ethanol & Biofuels Report. Available online: https://www.agra-net.com/agra/world-ethanol-and-biofuels-report/pdf-archive/article544385.ece/BINARY/World+Ethanol+%26+Biofuels+Report (accessed on 3 July 2019).
- Le, N.L.; Wang, Y.; Chung, T.S. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J. Membr. Sci. 2011, 379, 174–183. [Google Scholar] [CrossRef]
- Garhyan, P.; Elnashaie, S. Utilization of mathematical models to investigate the bifurcation and chaotic behavior of ethanol fermentors. Math. Comput. Model. 2004, 39, 381–427. [Google Scholar] [CrossRef]
- Utama, G.L.; Kurnani, T.B.A.; Balia, R.L. The isolation and identification of stress tolerance ethanol-fermenting yeasts from Mozzarella cheese whey. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 252–257. [Google Scholar] [CrossRef]
- Tian, M.; Row, K.H. Separation of glucose and bioethanol in biomass with current methods and sorbents. J. Chromatogr. Sci. 2013, 51, 819–824. [Google Scholar] [CrossRef]
- Lei, Z.; Li, C.; Chen, B. Extractive distillation: A review. Sep. Purif. Rev. 2003, 32, 121–213. [Google Scholar] [CrossRef]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Biorefin. 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Hilmioglu, N.D. Bioethanol Production by Pervaporation Membrane Bioreactor. J. Selcuk Univ. Nat. Appl. Sci. 2013, 2, 258–264. [Google Scholar]
- Serra, A.; Poch, M.; Sola, C. A survey of separation systems for fermentation ethanol recovery. Process. Biochem. 1987, 22, 154–158. [Google Scholar]
- Offeman, R.D.; Stephenson, S.K.; Robertson, G.H.; Orts, W.J. Solvent extraction of ethanol from aqueous solutions. I. Screening methodology for solvents. Ind. Eng. Chem. Res. 2005, 44, 6789–6796. [Google Scholar] [CrossRef]
- Brown, S.; Oliver, S.; Harrison, D.; Righelato, R. Ethanol inhibition of yeast growth and fermentation: Differences in the magnitude and complexity of the effect. Eur. J. Appl. Microbiol. Biotechnol. 1981, 11, 151–155. [Google Scholar] [CrossRef]
- Luong, J. Kinetics of ethanol inhibition in alcohol fermentation. Biotechnol. Bioeng. 1985, 27, 280–285. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef]
- Aiba, S.; Shoda, M.; Nagatani, M. Kinetics of product inhibition in alcohol fermentation. Biotechnol. Bioeng. 2000, 67, 671–690. [Google Scholar] [CrossRef]
- Moulin, G.; Boze, H.; Galzy, P. Inhibition of alcoholic fermentation by substrate and ethanol. Biotechnol. Bioeng. 1980, 22, 2375–2381. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, L.; Shen, J.; Shi, J.; Chen, G.; Zhao, J.; Duan, J.; Liu, G.; Jin, W. Improved ethanol recovery through mixed-matrix membrane with hydrophobic MAF-6 as filler. Sep. Purif. Technol. 2017, 178, 105–112. [Google Scholar] [CrossRef]
- De Vrije, T.; Budde, M.; van der Wal, H.; Claassen, P.A.; López-Contreras, A.M. “In situ” removal of isopropanol, butanol and ethanol from fermentation broth by gas stripping. Bioresour. Technol. 2013, 137, 153–159. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Auresenia, J.; Kosuge, H.; Tan, R.R.; Brondial, Y. Vacuum fermentation integrated with separation process for ethanol production. Biochem. Eng. J. 2011, 55, 208–214. [Google Scholar] [CrossRef]
- Offeman, R.D.; Stephenson, S.K.; Franqui, D.; Cline, J.L.; Robertson, G.H.; Orts, W.J. Extraction of ethanol with higher alcohol solvents and their toxicity to yeast. Sep. Purif. Technol. 2008, 63, 444–451. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
- Noble, R.D.; Stern, S.A. Membrane Separations Technology: Principles and Applications, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Choi, H.; Zhang, K.; Dionysiou, D.D.; Oerther, D.B.; Sorial, G.A. Influence of cross-flow velocity on membrane performance during filtration of biological suspension. J. Membr. Sci. 2005, 248, 189–199. [Google Scholar] [CrossRef]
- Hassan, I.; Ennouri, M.; Lafforgue, C.; Schmitz, P.; Ayadi, A. Experimental study of membrane fouling during crossflow microfiltration of yeast and bacteria suspensions: Towards an analysis at the microscopic level. Membranes 2013, 3, 44–68. [Google Scholar] [CrossRef]
- Xue, C.; Yang, D.; Du, G.; Chen, L.; Ren, J.; Bai, F. Evaluation of hydrophobic micro-zeolite-mixed matrix membrane and integrated with acetone–butanol–ethanol fermentation for enhanced butanol production. Biotechnol. Biofuels 2015, 8, 105. [Google Scholar] [CrossRef]
- Cath, T.Y.; Elimelech, M.; McCutcheon, J.R.; McGinnis, R.L.; Achilli, A.; Anastasio, D.; Brady, A.R.; Childress, A.E.; Farr, I.V.; Hancock, N.T. Standard methodology for evaluating membrane performance in osmotically driven membrane processes. Desalination 2013, 312, 31–38. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Tang, S. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor. Water Res. 2009, 43, 2504–2512. [Google Scholar] [CrossRef]
- Yeom, C.; Huang, R. Modelling of the pervaporation separation of ethanol-water mixtures through crosslinked poly (vinyl alcohol) membrane. J. Membr. Sci. 1992, 67, 39–55. [Google Scholar] [CrossRef]
- Lipnizki, F.; Trägårdh, G. Modelling of pervaporation: Models to analyze and predict the mass transport in pervaporation. Sep. Purif. Methods 2001, 30, 49–125. [Google Scholar] [CrossRef]
- Ebneyamini, A.; Azimi, H.; Tezel, F.H.; Thibault, J. Modelling of mixed matrix membranes: Validation of the resistance-based model. J. Membr. Sci. 2017, 543, 361–369. [Google Scholar] [CrossRef]
- Fan, S.; Xiao, Z.; Li, M.; Li, S. Pervaporation membrane bioreactor with permeate fractional condensation and mechanical vapor compression for energy efficient ethanol production. Appl. Energy 2016, 179, 939–947. [Google Scholar] [CrossRef]
- Kaewkannetra, P.; Chutinate, N.; Moonamart, S.; Kamsan, T.; Chiu, T.Y. Experimental study and cost evaluation for ethanol separation from fermentation broth using pervaporation. Desalin. Water Treat. 2012, 41, 88–94. [Google Scholar] [CrossRef]
- Gaykawad, S.S.; Zha, Y.; Punt, P.J.; van Groenestijn, J.W.; van der Wielen, L.A.; Straathof, A.J. Pervaporation of ethanol from lignocellulosic fermentation broth. Bioresour. Technol. 2013, 129, 469–476. [Google Scholar] [CrossRef]
- Bello, R.H.; Linzmeyer, P.; Franco, C.M.B.; Souza, O.; Sellin, N.; Medeiros, S.H.W.; Marangoni, C. Pervaporation of ethanol produced from banana waste. Waste Manag. 2014, 34, 1501–1509. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wei, P.; Zhang, L.; Huang, H. Pervaporation behavior and integrated process for concentrating lignocellulosic ethanol through polydimethylsiloxane (PDMS) membrane. Bioprocess. Biosyst. Eng. 2014, 37, 183–191. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Cho, E.J.; Lee, Y.J.; Bae, H.J.; Lee, H.J. Pervaporative separation of bioethanol produced from the fermentation of waste newspaper. J. Ind. Eng. Chem. 2013, 19, 1910–1915. [Google Scholar] [CrossRef]
- Cai, D.; Hu, S.; Chen, C.; Wang, Y.; Zhang, C.; Miao, Q.; Qin, P.; Tan, T. Immobilized ethanol fermentation coupled to pervaporation with silicalite-1/polydimethylsiloxane/polyvinylidene fluoride composite membrane. Bioresour. Technol. 2016, 220, 124–131. [Google Scholar] [CrossRef]
- Outram, V.; Lalander, C.A.; Lee, J.G.; Davies, E.T.; Harvey, A.P. Applied in situ product recovery in ABE fermentation. Biotechnol. Prog. 2017, 33, 563–579. [Google Scholar] [CrossRef]
- Ezeji, T.; Qureshi, N.; Blaschek, H. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Liu, H.S.; Hus, H.W. Analysis of gas stripping during ethanol fermentation—I. In a continuous stirred tank reactor. Chem. Eng. Sci. 1990, 45, 1289–1299. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.J.; Liu, D.H. Effect of different types of gas in gas stripping ethanol fermentation (GSEF). Chin. J. Process. Eng. 2005, 5, 349. [Google Scholar]
- Silva, C.; Esperança, M.; Cruz, A.; Moura, L.; Badino, A. Stripping of ethanol with CO2 in bubble columns: Effects of operating conditions and modeling. Chem. Eng. Res. Des. 2015, 102, 150–160. [Google Scholar] [CrossRef]
- Taylor, F.; Kurantz, M.J.; Goldberg, N.; Craig, J.C. Continuous fermentation and stripping of ethanol. Biotechnol. Prog. 1995, 11, 693–698. [Google Scholar] [CrossRef]
- Taylor, F.; Kurantz, M.J.; Goldberg, N.; Craig, J.C. Effects of ethanol concentration and stripping temperature on continuous fermentation rate. Appl. Microbiol. Biotechnol. 1997, 48, 311–316. [Google Scholar] [CrossRef]
- Sonego, J.; Lemos, D.; Cruz, A.; Badino, A. Optimization of Fed-Batch Fermentation with in Situ Ethanol Removal by CO2 Stripping. Energy Fuels 2017, 32, 954–960. [Google Scholar] [CrossRef]
- Andersen, R.L.; Jensen, K.M.; Mikkelsen, M.J. Continuous ethanol fermentation of pretreated lignocellulosic biomasses, waste biomasses, molasses and syrup using the anaerobic, thermophilic bacterium Thermoanaerobacter italicus pentocrobe 411. PLoS ONE 2015, 10, e0136060. [Google Scholar] [CrossRef]
- Sonego, J.L.; Lemos, D.A.; Rodriguez, G.Y.; Cruz, A.J.; Badino, A.C. Extractive batch fermentation with CO2 stripping for ethanol production in a bubble column bioreactor: Experimental and modeling. Energy Fuels 2014, 28, 7552–7559. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H.; Dai, S.H. A novel solid state fermentation coupled with gas stripping enhancing the sweet sorghum stalk conversion performance for bioethanol. Biotechnol. Biofuels 2014, 7, 53. [Google Scholar] [CrossRef]
- Ponce, G.H.S.F.; Neto, J.M.; De Jesus, S.S.; de Carvalho Miranda, J.C.; Maciel Filho, R.; de Andrade, R.R.; Maciel, M.R.W. Sugarcane molasses fermentation with in situ gas stripping using low and moderate sugar concentrations for ethanol production: Experimental data and modeling. Biochem. Eng. J. 2016, 110, 152–161. [Google Scholar] [CrossRef]
- Strods, M.; Mezule, L. Alcohol recovery from fermentation broth with gas stripping: System experimental and optimisation. Agron. Res. 2017, 15, 897–904. [Google Scholar]
- Cysewski, G.R.; Wilke, C.R. Rapid ethanol fermentations using vacuum and cell recycle. Biotechnol. Bioeng. 1977, 19, 1125–1143. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Qureshi, N.; Chen, M.H.; Liu, W.; Singh, V. Ethanol production from food waste at high solids content with vacuum recovery technology. J. Agric. Food Chem. 2015, 63, 2760–2766. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Kosuge, H.; Auresenia, J.; Tan, R.; Brondial, Y. Effect of vacuum pressure on ethanol fermentation. J. Appl. Sci. 2009, 9, 3020–3026. [Google Scholar] [CrossRef]
- Abdullah, A.; Ariyanti, D. Enhancing Ethanol production by fermentation using Saccharomyces cereviseae under vacuum condition in batch operation. Int. J. Renew. Energy Dev. 2012, 1, 6–9. [Google Scholar] [CrossRef]
- Samnuknit, W.; Boontawan, A. Extractive fermentation of ethanol using vacuum fractionation technique. Int. J. Chem. Nucl. Mater. Metall. Eng. 2014, 8, 456–464. [Google Scholar]
- Shihadeh, J.K.; Huang, H.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Vacuum stripping of ethanol during high solids fermentation of corn. Appl. Biochem. Biotechnol. 2014, 173, 486–500. [Google Scholar] [CrossRef]
- Ghose, T.; Roychoudhury, P.; Ghosh, P. Simultaneous saccharification and fermentation (SSF) of lignocellulosics to ethanol under vacuum cycling and step feeding. Biotechnol. Bioeng. 1984, 26, 377–381. [Google Scholar] [CrossRef]
- Phakping, S.; Ketudat-Cairns, M.; Boontawan, A. Extractive Fermentation of Ethanol from Fresh Cassava Roots Using Vacuum Fractionation Technique. Adv. Mater. Res. 2014, 931–932, 1096–1100. [Google Scholar] [CrossRef]
- Kongkaew, A.; Tönjes, J.; Siemer, M.; Boontawan, P.; Rarey, J.; Boontawan, A. Extractive Fermentation of Ethanol from Sweet Sorghum Using Vacuum Fractionation Technique: Optimization and Techno-Economic Assessment. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Fujita, H.; Qian, Q.; Fujii, T.; Mochizuki, K.; Sakoda, A. Isolation of ethanol from its aqueous solution by liquid phase adsorption and gas phase desorption using molecular sieving carbon. Adsorption 2011, 17, 869–879. [Google Scholar] [CrossRef]
- Bui, S.; Verykios, X.; Mutharasan, R. In situ removal of ethanol from fermentation broths. 1. Selective adsorption characteristics. Ind. Eng. Chem. Process. Des. Dev. 1985, 24, 1209–1213. [Google Scholar] [CrossRef]
- Walsh, P.; Liu, C.; Findley, M.; Liapis, A.; Siehr, D. Ethanol separation from water in a two-stage adsorption process. Biotechnol. Bioeng. Symp. 1983, 13, 629–647. [Google Scholar]
- Hashi, M.; Tezel, F.H.; Thibault, J. Ethanol recovery from fermentation broth via carbon dioxide stripping and adsorption. Energy Fuels 2010, 24, 4628–4637. [Google Scholar] [CrossRef]
- Saha, B.; El-Sharkawy, I.; Chakraborty, A.; Koyama, S. Study on an activated carbon fiber–ethanol adsorption chiller: Part I–system description and modelling. Int. J. Refrig. 2007, 30, 86–95. [Google Scholar] [CrossRef]
- Nguyen, C.; Sonwane, C.; Bhatia, S.; Do, D. Adsorption of benzene and ethanol on MCM-41 material. Langmuir 1998, 14, 4950–4952. [Google Scholar] [CrossRef]
- Rebar, V.; Fischbach, E.; Apostolopoulos, D.; Kokini, J. Thermodynamics of water and ethanol adsorption on four starches as model biomass separation systems. Biotechnol. Bioeng. 1984, 26, 513–517. [Google Scholar] [CrossRef]
- Yang, J.Z.; Liu, Q.L.; Wang, H.T. Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J. Membr. Sci. 2007, 291, 1–9. [Google Scholar] [CrossRef]
- Carton, A.; Benito, G.G.; Rey, J.; de La Fuente, M. Selection of adsorbents to be used in an ethanol fermentation process. Adsorption isotherms and kinetics. Bioresour. Technol. 1998, 66, 75–78. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Miyazaki, T.; Koyama, S.; Henninger, S.K.; Herbst, A.; Janiak, C. Ethanol adsorption onto metal organic framework: Theory and experiments. Energy 2015, 79, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Caputo, D.; Iucolano, F.; Pepe, F.; Colella, C. Modeling of water and ethanol adsorption data on a commercial zeolite-rich tuff and prediction of the relevant binary isotherms. Microporous Mesoporous Mater. 2007, 105, 260–267. [Google Scholar] [CrossRef]
- Oudshoorn, A.; van der Wielen, L.A.; Straathof, A.J. Adsorption equilibria of bio-based butanol solutions using zeolite. Biochem. Eng. J. 2009, 48, 99–103. [Google Scholar] [CrossRef]
- Jones, R.; Gandier, J.; Thibault, J.; Tezel, F. Enhanced ethanol production through selective adsorption in bacterial fermentation. Biotechnol. Bioprocess. Eng. 2011, 16, 531–541. [Google Scholar] [CrossRef]
- Seo, D.J.; Takenaka, A.; Fujita, H.; Mochidzuki, K.; Sakoda, A. Practical considerations for a simple ethanol concentration from a fermentation broth via a single adsorptive process using molecular-sieving carbon. Renew. Energy 2018, 118, 257–264. [Google Scholar] [CrossRef]
- Minier, M.; Coma, G. Production of ethanol by coupling fermentation and solvent extraction. Biotechnol. Lett. 1981, 3, 405–408. [Google Scholar] [CrossRef]
- Kollerup, F.; Daugulis, A.J. Ethanol production by extractive fermentation-solvent identification and prototype development. Can. J. Chem. Eng. 1986, 64, 598–606. [Google Scholar] [CrossRef]
- Kollerup, F.; Daugulis, A.J. Screening and identification of extractive fermentation solvents using a database. Can. J. Chem. Eng. 1985, 63, 919–927. [Google Scholar] [CrossRef]
- Matsumura, M.; Märkl, H. Application of solvent extraction to ethanol fermentation. Appl. Microbiol. Biotechnol. 1984, 20, 371–377. [Google Scholar] [CrossRef]
- Job, C.; Schertler, C.; Staudenbauer, W.L.; Blass, E. Selection of organic solvents for in situ extraction of fermentation products fromClostridium thermohydrosulfuricum cultures. Biotechnol. Tech. 1989, 3, 315–320. [Google Scholar] [CrossRef]
- Honda, H.; Taya, M.; Kobayashi, T. Ethanol fermentation associated with solvent extraction using immobilized growing cells of Saccharomyces cerevisiae and its lactose-fermentable fusant. J. Chem. Eng. Jpn. 1986, 19, 268–273. [Google Scholar] [CrossRef]
- Barros, M.A.; Cabral, J.; Novais, J. Production of ethanol by immobilized Saccharomyces bayanus in an extractive fermentation system. Biotechnol. Bioeng. 1987, 29, 1097–1104. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-economic analysis of bioethanol production from lignocellulosic biomass in China: Dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Kim, H.; Lee, J.Y.; Yoon, M.H.; Won, S.; Lee, B.C.; Song, K.G. Membrane fouling control using a rotary disk in a submerged anaerobic membrane sponge bioreactor. Bioresour. Technol. 2014, 172, 321–327. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Vane, L.M.; Alvarez, F.R.; Rosenblum, L.; Govindaswamy, S. Efficient ethanol recovery from yeast fermentation broth with integrated distillation-membrane process. Ind. Eng. Chem. Res. 2012, 52, 1033–1041. [Google Scholar] [CrossRef]
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Hemra, K.; Kulprathipanja, S.; James, R.; Wongkasemjit, S. Techno-economic comparison of energy usage between azeotropic distillation and hybrid system for water-ethanol separation. Renew. Energy 2013, 51, 310–316. [Google Scholar] [CrossRef]






| Membrane Materials | Feedstock | Operating Conditions | Separation Factor | Total Flux (Kg/m2h) | Ref |
|---|---|---|---|---|---|
| Cellulose acetate/PVA | Sorghum juice | 50 °C, 50.8 mm Hg, 14.8 wt%., NA | 6.6 | 2.7 | [40] |
| PDMS | Barley straw | 30 °C, 15 mm Hg, 15 wt%,950 g/min | 4.2 | 0.567 | [41] |
| PDMS | Willow wood chips | 30 °C, 15 mm Hg, 15 wt%,950 g/min | 4.8 | 0.498 | [41] |
| PDMS | Banana waste | 25 °C, 19.5 mm Hg, 3.8 wt%,19.8 L/h | 10.36 | 0.001 | [42] |
| PDMS/silicalite | Lignocellulosic | 40 °C, 1.05 mm Hg, 5 wt%,1.5 L/min | 11.6 | 0.291 | [43] |
| PDMS | Glucose | 40 °C, 5 mm Hg, 4.7 wt%,1.8 L/min | 9.0 | 0.049 | [44] |
| PDMS | Newspaper waste | 40 °C, 5 mm Hg, 4 wt%,1.8 L/min | 7.9 | 0.050 | [44] |
| Silicalite /PDMS /PVDF | Sweet sorghum stem | 30 °C, 0.75 mm Hg, 11%,1.5 L/min | 9.8 | 0.395 | [45] |
| Stripping Gas | Aim of the Study | Findings | Ref |
|---|---|---|---|
| Carbon dioxide | Integration of continuous fermentation and stripping of ethanol | Biomass yield was lower than in a simple continuous fermentor 150 days of operation without contamination. | [51] |
| Carbon dioxide | Effects of ethanol concentration and stripping temperature on continuous fermentation rate. | Fermentation rate was linearly related to the ethanol concentration and stripping temperature. | [52] |
| Carbon dioxide | Optimization of Fed-Batch fermentation coupled with gas stripping | Integration of gas stripping increased ethanol production by 65%. Fermentation using high sucrose concentration (up to 428.6 g L−1) was usefully achieved with ethanol removal. | [53] |
| Nitrogen | Continuous ethanol fermentation of pretreated lignocellulosic biomasses with gas stripping | The yield was 0.47–0.49 g/g close to maximum theoretical yield with 1.2–2.7 g/L/h volumetric ethanol productivity and 90–99% sugar conversion. | [54] |
| Carbon dioxide | Extractive batch fermentation with CO2 stripping for ethanol production | Optimal conditions for ethanol stripping was 2.0 vvm of CO2 flow rate and 34 °C of feed temperature. 25% higher productivity was obtained after 3 hours of stripping. | [55] |
| Carbon dioxide | Enhancing sweet sorghum state fermentation by gas stripping | Optimal conditions were 10 h of gas stripping time, 35 °C gas stripping temperature, 28 h of fermentation, and 0.15 cm particle thickness of GS-SSF. 6% to 10% increase of ethanol yield with gas stripping. | [56] |
| Carbon dioxide | Sugarcane molasses fermentation with in situ gas stripping | Ethanol concentration was kept below the threshold of toxicity (<60 g/L). A mathematical model was developed to describe the system. | [57] |
| Nitrogen, Carbon dioxide | Optimization of alcohol recovery from fermentation broth with gas stripping | 60 L·min−1 flow rate and 10 wt% ethanol concentration were the optimal conditions for efficient recovery Carbon dioxide stripping did not show recovery potential at low ethanol concentrations. | [58] |
| Parameters | Aim of the Study | Findings | Ref |
|---|---|---|---|
| Glucose C = 250 g/L P = 47–760 mm Hg, T = 33 °C | Effect of vacuum pressure on ethanol fermentation | 33.2 wt% ethanol condensate was collected at the outlet. A model to predict the kinetic parameters for the fermentation under the vacuum was developed. | [61] |
| Glucose C = 100 g/L P = 65-700 mm Hg, T = 30 °C | Effect of pressure and glucose concentration in ethanol fermentation | Vacuum condition can increase yeast growth and ethanol productivity. The optimal pressure was 36.6 mmHg. | [62] |
| Glucose C = 25 g/L P = 48.75 mm Hg T = 35 °C | Investigation of extractive fermentation by using a vacuum fractionation technique | High substrate utilization rate was obtained at 26.6 g/L h. Ethanol production increased more than 8- fold higher compared to batch fermentation. | [63] |
| Corn C = 450 g/L P = 711.2 mm Hg T = 32 °C | Effects of vacuum application on improving ethanol yield during high solids fermentation | 30% increase in ethanol yield after vacuum application. | [64] |
| Rice straw C = 100 g/L P = 80 mm Hg T = 40 °C | Effect vacuum cycling on simultaneous saccharification and fermentation (SSF) for ethanol production | Increase with 4 folds in the ethanol productivity in SSF after using vacuum cycling. | [65] |
| Cassava root C = 100 g/L P = 80 mm Hg T = 40 °C | Integration of vacuum fractionation technique in extractive fermentation from ethanol production from fresh cassava roots | The recovered ethanol concentration was around 86 wt%. The concentration of ethanol in the broth was kept below 2 wt%. | [66] |
| Sweet sorghum C = 0–600 g/L P = 48.75 mm Hg T = 35 °C | Optimization of vacuum fermentation and techno-economic assessment | 80 wt% ethanol concentration was continuously removed. Low production cost with time saving compared to the conventional fermentation. | [67] |
| Parameters | Solvents | Findings | Ref |
|---|---|---|---|
| S.cerevisiae Glucose C = 100 g/L T = 30 °C | 25 solvents (alcohols and esters) | Most of these solvents were toxic to the ethanol-producing microorganism. Immobilizing the cells using Porapack Q showed effective protection against solvent toxicity. | [85] |
| S.cerevisiae Glucose C = 407 g/L T = 35 °C | Dodecanol | Ethanol productivity was 5 fold higher than the productivity achieved in conventional fermentation. | [82] |
| C.thermohydrosulfuricum NA C = NA T = 65 °C | Hexadecane, isooctane, kerosene, oleyl alcohol, Shellsol TD | With excepting the kerosene, all solvents showed high ethanol separation from the aqueous phase. Oleyl alcohol showed the maximum partition coefficient for ethanol((KD = 0.34). | [86] |
| S.cerevisiae Glucose C = 100 g/L T = 30 °C | O-isopropylphenol (OIPP) O-tert-butylphenol (OTBP) | Both solvents have shown high partition coefficients for ethanol but they have heavy toxicity. Immobilizing of the yeast cells using alginate gel together limit the toxicity of both solvents. | [87] |
| S. bayanus Glucose C = 300 g/L T = 35 °C | Valeric, Hexanoic, Octanoic, Oleic | Oleic was the best selective solvent. Immobilizing the cells using k-carrageenan essentially was protective against solvent toxicity. The highest productivity was 1.39 g/L/h at 5:1 oleic acid fermentation medium ratio. | [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zentou, H.; Abidin, Z.Z.; Yunus, R.; Awang Biak, D.R.; Korelskiy, D. Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth. Processes 2019, 7, 458. https://doi.org/10.3390/pr7070458
Zentou H, Abidin ZZ, Yunus R, Awang Biak DR, Korelskiy D. Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth. Processes. 2019; 7(7):458. https://doi.org/10.3390/pr7070458
Chicago/Turabian StyleZentou, Hamid, Zurina Zainal Abidin, Robiah Yunus, Dayang Radiah Awang Biak, and Danil Korelskiy. 2019. "Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth" Processes 7, no. 7: 458. https://doi.org/10.3390/pr7070458