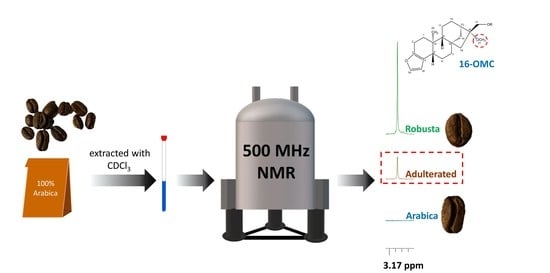
Authentication of Coffee Blends by 16-O-Methylcafestol Quantification Using NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Coffee samples
2.3. Sample Treatment
2.4. NMR Spectroscopy
2.5. Quantification
= (A16-OMC/N16-OMC) × (NI.S. × WI.S./AI.S. × MWI.S. × VS)
= [(C16-OMC × V × MW16-OMC) / (1000 × WS)] × 1000
2.6. Validation
2.7. Statistical Analysis
3. Results and Discussion
3.1. 1H NMR Spectra of Coffee Extracts
3.2. The Free Form and Esterified of 16-OMC
3.3. The Decomposition Products of 16-OMC
3.4. Validation
3.5. Surveillance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Ruge, W.; Kuballa, T.; Ilse, M.; Winkelmann, O.; Diehl, B.; Thomas, F.; Lachenmeier, D.W. Rapid approach to identify the presence of Arabica and Robusta species in coffee using 1H NMR spectroscopy. Food Chem. 2015, 182, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef] [Green Version]
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee adulteration: More than two decades of research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Finotello, C.; Forzato, C.; Gasparini, A.; Mammi, S.; Navarini, L.; Schievano, E. NMR quantification of 16-O-methylcafestol and kahweol in Coffea canephora var. robusta beans from different geographical origins. Food Control. 2017, 75, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Valdenebro, M.S.; Lelejn-Camacho, M.; Pablos, F.; González, A.G.; Martin, M.J. Determination of the arabica/robusta composition of roasted coffee according to their sterolic content. Analyst 1999, 124, 999–1002. [Google Scholar] [CrossRef]
- Schievano, E.; Finotello, C.; De Angelis, E.; Mammi, S.; Navarini, L. Rapid authentication of coffee blends and quantification of 16-O-methylcafestol in roasted coffee beans by nuclear magnetic resonance. J. Agric. Food Chem. 2014, 62, 12309–12314. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Marín, M.J.; Pablos, F.; González, A.G.; Valdenebro, M.S.; León-Camacho, M. Fatty acid profiles as discriminant parameters for coffee varieties differentiation. Talanta 2001, 54, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.R.; da Silva, C.C.; Garrett, R.; Rezende, C.M. Comprehensive lipid analysis of green Arabica coffee beans by LC-HRMS/MS. Food Res. Int. 2020, 137, 109727. [Google Scholar] [CrossRef]
- Guercia, E.; Colomban, S.; Navarini, L. 16-O-Methylated diterpenes in green Coffea arabica: Ultra-high-performance liquid chromatography-tandem mass spectrometry method optimization and validation. J. Mass Spectrom. 2020, 55, e4636. [Google Scholar] [CrossRef] [PubMed]
- Portaluri, V.; Thomas, F.; Guyader, S.; Jamin, E.; Bertrand, B.; Remaud, G.S.; Schievano, E.; Mammi, S.; Guercia, E.; Navarini, L. Limited genotypic and geographic variability of 16-O-methylated diterpene content in Coffea arabica green beans. Food Chem. 2020, 329, 127129. [Google Scholar] [CrossRef]
- Standard DIN 10779; Analysis of Coffee and Coffee Products, Determination of 16-O-Methyl Cafestol Content of Roasted Coffee HPLC-Method. DIN (n.d.): Berlin, Germany, 2011.
- Defernez, M.; Wren, E.; Watson, A.D.; Gunning, Y.; Colquhoun, I.J.; Le Gall, G.; Williamson, D.; Kemsley, E.K. Low-field 1H NMR spectroscopy for distinguishing between arabica and robusta ground roast coffees. Food Chem. 2017, 216, 106–113. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, N.; De Angelis, E.; Navarini, L.; Schievano, E.; Mammi, S. Green coffee oil analysis by high-resolution nuclear magnetic resonance spectroscopy. Talanta 2013, 110, 118–127. [Google Scholar] [CrossRef]
- Okaru, A.O.; Scharinger, A.; Rajcic de Rezende, T.; Teipel, J.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. Validation of a quantitative proton nuclear magnetic resonance spectroscopic screening method for coffee quality and authenticity (NMR coffee screener). Foods 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacetti, D.; Boselli, E.; Balzano, M.; Frega, N.G. Authentication of Italian Espresso coffee blends through the GC peak ratio between kahweol and 16-O-methylcafestol. Food Chem. 2012, 135, 1569–1574. [Google Scholar] [CrossRef]
- Wermelinger, T.; D’Ambrosio, L.; Klopprogge, B.; Yeretzian, C. Quantification of the Robusta fraction in a coffee blend via Raman spectroscopy: Proof of principle. J. Agric. Food Chem. 2011, 59, 9074–9079. [Google Scholar] [CrossRef] [PubMed]
- Keidel, A.; von Stetten, D.; Rodrigues, C.; Máguas, C.; Hildebrandt, P. Discrimination of green Arabica and Robusta coffee beans by Raman spectroscopy. J. Agric. Food Chem. 2010, 58, 11187–11192. [Google Scholar] [CrossRef] [PubMed]
- Assis, C.; Pereira, H.V.; Amador, V.S.; Augusti, R.; de Oliveira, L.S.; Sena, M.M. Combining mid infrared spectroscopy and paper spray mass spectrometry in a data fusion model to predict the composition of coffee blends. Food Chem. 2019, 281, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Navarini, L.; Guercia, E.; Oreški, A.; Gasparini, A.; Scoltock, J.; Forzato, C. Interaction of the coffee diterpenes cafestol and 16-O-methyl-cafestol palmitates with serum albumins. Int. J. Mol. Sci. 2020, 21, 1823. [Google Scholar] [CrossRef] [Green Version]
- Moeenfard, M.; Erny, G.L.; Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J. Food Sci. Technol. 2016, 53, 3916–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novaes, F.J.M.; Oigman, S.S.; de Souza, R.O.M.A.; Rezende, C.M.; de Aquino Neto, F.R. New approaches on the analyses of thermolabile coffee diterpenes by gas chromatography and its relationship with cup quality. Talanta 2015, 139, 159–166. [Google Scholar] [CrossRef]
- Gross, G.; Jaccaud, E.; Huggett, A.C. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef]
- Moeenfard, M.; Silva, J.A.; Borges, N.; Santos, A.; Alves, A. Quantification of diterpenes and their palmitate esters in coffee brews by HPLC–DAD. Int. J. Food Prop. 2015, 18, 2284–2299. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lim, L.; Fu, Y. Review of analytical methods to detect adulteration in coffee. J. AOAC Int. 2020, 103, 295–305. [Google Scholar] [CrossRef]
- Mihailova, A.; Liebisch, B.; Islam, M.D.; Carstensen, J.M.; Cannavan, A.; Kelly, S.D. The use of multispectral imaging for the discrimination of Arabica and Robusta coffee beans. Food Chem. X 2022, 14, 100325. [Google Scholar] [CrossRef] [PubMed]
- Carrera, F.; León-Camacho, M.; Pablos, F.; González, A.G. Authentication of green coffee varieties according to their sterolic profile. Anal. Chim. Acta 1998, 370, 131–139. [Google Scholar] [CrossRef]
- Kamm, W.; Dionisi, F.; Fay, L.; Hischenhuber, C.; Schmarr, H.; Engel, K. Rapid and simultaneous analysis of 16-O-methylcafestol and sterols as markers for assessment of green coffee bean authenticity by on-line LC-GC. J. Am. Oil Chem. Soc. 2002, 79, 1109–1113. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Casale, R.; Cautela, D.; D’Onofrio, N.; Balestrieri, M.L.; Castaldo, D. Homostachydrine (pipecolic acid betaine) as authentication marker of roasted blends of Coffea arabica and Coffea canephora (Robusta) beans. Food Chem. 2016, 205, 52–57. [Google Scholar] [CrossRef]
- Romano, R.; Santini, A.; Le Grottaglie, L.; Manzo, N.; Visconti, A.; Ritieni, A. Identification markers based on fatty acid composition to differentiate between roasted Arabica and Canephora (Robusta) coffee varieties in mixtures. J. Food Compos. Anal. 2014, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]




| Sample Number | Country of Production | Labeled Species | 16-OMC (mg/kg) | SD |
|---|---|---|---|---|
| SB1 | Taiwan | 100% Arabica | nd a | - |
| SB2 | Taiwan | 100% Arabica | nd | - |
| SB3 | Taiwan | 100% Arabica | 426.50 | 27.50 |
| SB4 | Taiwan | 100% Arabica | 234.78 | 11.18 |
| SB5 | Taiwan | 100% Arabica | 368.75 | 12.50 |
| SB6 | Taiwan | 100% Arabica | nd | - |
| SB7 | Taiwan | 100% Arabica | nd | - |
| SB8 | Taiwan | 100% Arabica | nd | - |
| SB9 | Taiwan | 100% Arabica | nd | - |
| SB10 | Taiwan | 100% coffee | 617.61 | 66.87 |
| SB11 | Taiwan | 100% Arabica | 401.74 | 35.20 |
| SB12 | Taiwan | 100% Arabica | 155.74 | 9.43 |
| SB13 | Taiwan | 100% Arabica | 296.62 | 16.37 |
| SB14 | Taiwan | 100% Arabica | 222.62 | 31.32 |
| SB15 | Taiwan | 100% Arabica | 316.57 | 17.74 |
| SB16 | Taiwan | 100% Arabica | 207.96 | 0.83 |
| SB17 | Taiwan | 100% Arabica | 404.03 | 37.17 |
| SB18 | Taiwan | 100% coffee | 554.33 | 45.48 |
| SB19 | Taiwan | 100% coffee | 266.86 | 8.29 |
| SB20 | Taiwan | 100% Arabica | 276.30 | 12.58 |
| SB21 | Taiwan | 100% Arabica | 385.00 | 15.13 |
| SB22 | Taiwan | 100% Arabica | 247.35 | 0.74 |
| SB23 | Taiwan | 100% Arabica | 282.66 | 15.79 |
| SB24 | Taiwan | 100% Arabica | 784.60 | 60.05 |
| SB25 | Taiwan | 100% Arabica | 623.74 | 39.58 |
| SB26 | Taiwan | 100% Arabica | 722.94 | 60.31 |
| SB27 | Sweden | 100% Arabica | nd | - |
| SB28 | Italia | 100% Arabica | nd | - |
| SB29 | Italia | 100% Arabica | nd | - |
| SB30 | Colombia | 100% Arabica | nd | - |
| SB31 | Italia | Arabica | 214.56 | 10.58 |
| SB32 | Australia | 100% Arabica | nd | - |
| SB33 | Germany | 100% Arabica | nd | - |
| SB34 | Taiwan | 100% Arabica | nd | - |
| SB35 | Austria | Arabica | nd | - |
| SB36 | Australia | 100% Arabica | nd | - |
| SB37 | Taiwan | 100% Arabica | nd | - |
| SB38 | Taiwan | 100% Arabica | nd | - |
| SB39 | Taiwan | 100% Arabica | nd | - |
| SB40 | Taiwan | 100% Arabica | nd | - |
| SB41 | Taiwan | 100% Arabica | nd | - |
| SB42 | Taiwan | 100% Arabica | nd | - |
| SB43 | Taiwan | 100% Arabica | nd | - |
| SB44 | Taiwan | 100% Arabica | nd | - |
| SB45 | Taiwan | 100% Arabica | nd | - |
| SB46 | Taiwan | 100% Arabica | nd | - |
| SB47 | Taiwan | 100% Arabica | nd | - |
| SB48 | Taiwan | 100% Arabica | nd | - |
| SB49 | Taiwan | 100% Arabica | nd | - |
| SB50 | Taiwan | 100% Arabica | nd | - |
| SB51 | Taiwan | 100% Arabica | nd | - |
| SB52 | Taiwan | 100% Arabica | nd | - |
| Concentration of Spiked 16-OMC (mg/kg) | Recovery% | CV% |
|---|---|---|
| 50 | 108.9 | 6.4 |
| 100 | 103.5 | 3.0 |
| 500 | 81.9 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; We, Y.-L.; Kao, Y.-M.; Tseng, S.-H.; Wang, D.-Y.; Chen, S.-Y. Authentication of Coffee Blends by 16-O-Methylcafestol Quantification Using NMR Spectroscopy. Processes 2023, 11, 871. https://doi.org/10.3390/pr11030871
Lin Y-T, We Y-L, Kao Y-M, Tseng S-H, Wang D-Y, Chen S-Y. Authentication of Coffee Blends by 16-O-Methylcafestol Quantification Using NMR Spectroscopy. Processes. 2023; 11(3):871. https://doi.org/10.3390/pr11030871
Chicago/Turabian StyleLin, Ya-Tze, You-Lun We, Ya-Min Kao, Su-Hsiang Tseng, Der-Yuan Wang, and Shin-Yu Chen. 2023. "Authentication of Coffee Blends by 16-O-Methylcafestol Quantification Using NMR Spectroscopy" Processes 11, no. 3: 871. https://doi.org/10.3390/pr11030871