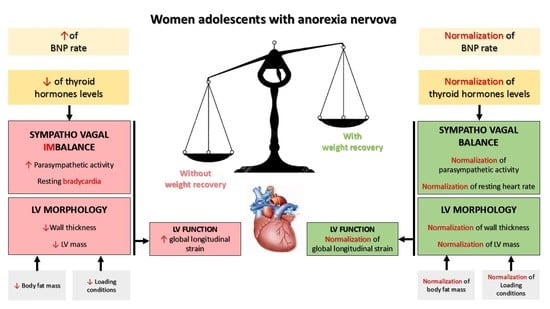
Cardiac Remodeling and Its Determinants in Anorexia Nervosa Adolescents: Impact of Weight Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometrical Data and Body Composition
2.3. Biological Data
2.4. Heart Rate Variability
2.5. Echocardiographic Evaluation
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Thyroid Axis and NT-proBNP
3.3. Sympatho-Vagal Balance
3.4. Left Ventricular Morphology and Function
3.5. Determinants of Sympatho-Vagal Balance
3.6. Determinants of LVM and GLS
4. Discussion
4.1. Sympatho-Vagal Balance and Resting Bradycardia in AN Patients without or with WR
4.2. Determinants of Sympatho-Vagal Imbalance and Resting Bradycardia
4.3. Left Ventricular Remodeling in AN Patients without or with WR
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivares, J.L.; Vázquez, M.; Fleta, J.; Moreno, L.A.; Pérez-González, J.M.; Bueno, M. Cardiac Findings in Adolescents with Anorexia Nervosa at Diagnosis and after Weight Restoration. Eur. J. Pediatr. 2005, 164, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Mont, L.; Castro, J.; Herreros, B.; Paré, C.; Azqueta, M.; Magriña, J.; Puig, J.; Toro, J.; Brugada, J. Reversibility of Cardiac Abnormalities in Adolescents With Anorexia Nervosa After Weight Recovery. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Galetta, F.; Franzoni, F.; Prattichizzo, F.; Rolla, M.; Santoro, G.; Pentimone, F. Heart Rate Variability and Left Ventricular Diastolic Function in Anorexia Nervosa. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2003, 32, 416–421. [Google Scholar]
- Gottdiener, J.S.; Gross, H.A.; Henry, W.L.; Borer, J.S.; Ebert, M.H. Effects of Self-Induced Starvation on Cardiac Size and Function in Anorexia Nervosa. Circulation 1978, 58, 425–433. [Google Scholar] [PubMed] [Green Version]
- Galetta, F.; Franzoni, F.; Cupisti, A.; Morelli, E.; Santoro, G.; Pentimone, F. Early Detection of Cardiac Dysfunction in Patients with Anorexia Nervosa by Tissue Doppler Imaging. Int. J. Cardiol. 2005, 101, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.A.; Potts, J.E.; Lam, P.-Y.; De Souza, A.M.; Mugford, G.J.; Sandor, G.G.S. An Echocardiographic Study of Left Ventricular Size and Cardiac Function in Adolescent Females with Anorexia Nervosa: LV Size and Cardiac Function in AN. Eur. Eat. Disord. Rev. 2016, 24, 26–33. [Google Scholar] [CrossRef]
- Kastner, S.; Salbach-Andrae, H.; Renneberg, B.; Pfeiffer, E.; Lehmkuhl, U.; Schmitz, L. Echocardiographic Findings in Adolescents with Anorexia Nervosa at Beginning of Treatment and after Weight Recovery. Eur. Child Adolesc. Psychiatry 2012, 21, 15–21. [Google Scholar] [CrossRef]
- Powers, P.S.; Schocken, D.D.; Feld, J.; Holloway, D.; Boyd, F. Cardiac Function during Weight Restoration in Anorexia Nervosa. Int. J. Eat. Disord. 1991, 10, 521–530. [Google Scholar]
- Morris, R.; Prasad, A.; Asaro, J.; Guzman, M.; Sanders, L.; Hauck, A.; Singh, G.K.; Levy, P.T. Markers of Cardiovascular Dysfunction in Adolescents With Anorexia Nervosa. Glob. Pediatr. Health 2017, 4, 2333794X17727423. [Google Scholar] [CrossRef]
- Spaulding-Barclay, M.A.; Stern, J.; Mehler, P.S. Cardiac Changes in Anorexia Nervosa. Cardiol. Young 2016, 26, 623–628. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Yamada, U.; Ohta, D. Low Body Mass Index Correlates with Low Left Ventricular Mass Index in Patients with Severe Anorexia Nervosa. Heart Vessels 2018, 33, 89–93. [Google Scholar] [CrossRef] [PubMed]
- St John Sutton, M.G.; Plappert, T.; Crosby, L.; Douglas, P.; Mullen, J.; Reichek, N. Effects of Reduced Left Ventricular Mass on Chamber Architecture, Load, and Function: A Study of Anorexia Nervosa. Circulation 1985, 72, 991–1000. [Google Scholar] [PubMed] [Green Version]
- Silvetti, M.S.; Magnani, M.; Santilli, A.; Di Liso, G.; Diamanti, A.; Pompei, E.; Gambarara, M.; Montecchi, F.; Ragonese, P. The heart of anorexic adolescents. G. Ital. Cardiol. 1998, 28, 131–139. [Google Scholar] [PubMed]
- Carlomagno, G.; Mercurio, V.; Ruvolo, A.; Senatore, I.; Halinskaya, I.; Fazio, V.; Affuso, F.; Fazio, S. Endocrine Alterations Are the Main Determinants of Cardiac Remodelling in Restrictive Anorexia Nervosa. ISRN Endocrinol. 2011, 2011, 171460. [Google Scholar] [CrossRef] [Green Version]
- Sachs, K.V.; Harnke, B.; Mehler, P.S.; Krantz, M.J. Cardiovascular Complications of Anorexia Nervosa: A Systematic Review. Int. J. Eat. Disord. 2016, 49, 238–248. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Bonelo-Perdomo, A.; Sierra-Torres, C.H. Effects of Thyroid Hormones on the Heart. Clínica Investig. Arterioscler. 2014, 26, 296–309. [Google Scholar] [CrossRef]
- Croxson, M.S.; Ibbertson, H.K. Low Serum Triiodothyronine (T3) and Hypothyroidism in Anorexia Nervosa. J. Clin. Endocrinol. Metab. 1977, 44, 167–174. [Google Scholar] [CrossRef]
- Muñoz, M.T.; Argente, J. Anorexia Nervosa in Female Adolescents: Endocrine and Bone Mineral Density Disturbances. Eur. J. Endocrinol. 2002, 147, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, K. Endocrine and reproductive disturbances in anorexia nervosa and bulimia nervosa. Nihon Rinsho Jpn. J. Clin. Med. 2001, 59, 549–553. [Google Scholar]
- Vila, G.; Grimm, G.; Resl, M.; Heinisch, B.; Einwallner, E.; Esterbauer, H.; Dieplinger, B.; Mueller, T.; Luger, A.; Clodi, M. B-Type Natriuretic Peptide Modulates Ghrelin, Hunger, and Satiety in Healthy Men. Diabetes 2012, 61, 2592–2596. [Google Scholar] [CrossRef] [Green Version]
- Costello-Boerrigter, L.C.; Burnett, J.C. A New Role for the Natriuretic Peptides. J. Am. Coll. Cardiol. 2009, 53, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, R.L. Cardioprotective Functions of Atrial Natriuretic Peptide and B-Type Natriuretic Peptide: A Brief Review. Clin. Exp. Pharmacol. Physiol. 2004, 31, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Kováts, T.; Tomcsányi, J. Bradycardia and B-Type Natriuretic Peptide. Int. J. Cardiol. 2009, 135, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Loas, G. The DSM-V: An overview. Rev. Med. Brux. 2016, 37, 231–234. [Google Scholar] [PubMed]
- Tj, C. The LMS Method for Constructing Normalized Growth Standards. Eur. J. Clin. Nutr. 1990, 44, 45–60. [Google Scholar]
- Orimadegun, A.; Omisanjo, A. Evaluation of Five Formulae for Estimating Body Surface Area of Nigerian Children. Ann. Med. Health Sci. Res. 2014, 4, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Barreira, T.V.; Staiano, A.E.; Katzmarzyk, P.T. Validity Assessment of a Portable Bioimpedance Scale to Estimate Body Fat Percentage in White and African-American Children and Adolescents. Pediatr. Obes. 2013, 8, e29–e32. [Google Scholar] [CrossRef] [Green Version]
- Giles, D.; Draper, N.; Neil, W. Validity of the Polar V800 Heart Rate Monitor to Measure RR Intervals at Rest. Eur. J. Appl. Physiol. 2016, 116, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Pichot, V.; Roche, F.; Celle, S.; Barthélémy, J.-C.; Chouchou, F. HRVanalysis: A Free Software for Analyzing Cardiac Autonomic Activity. Front. Physiol. 2016, 7, 557. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins, Methods, and Interpretive Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Billman, G.E. Heart Rate Variability—A Historical Perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic Assessment of Left Ventricular Hypertrophy: Comparison to Necropsy Findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef] [PubMed]
- de Simone, G.; Daniels, S.R.; Devereux, R.B.; Meyer, R.A.; Roman, M.J.; de Divitiis, O.; Alderman, M.H. Left Ventricular Mass and Body Size in Normotensive Children and Adults: Assessment of Allometric Relations and Impact of Overweight. J. Am. Coll. Cardiol. 1992, 20, 1251–1260. [Google Scholar]
- Nagueh, S.F.; Middleton, K.J.; Kopelen, H.A.; Zoghbi, W.A.; Quiñones, M.A. Doppler Tissue Imaging: A Noninvasive Technique for Evaluation of Left Ventricular Relaxation and Estimation of Filling Pressures. J. Am. Coll. Cardiol. 1997, 30, 1527–1533. [Google Scholar] [CrossRef]
- Maufrais, C.; Schuster, I.; Doucende, G.; Vitiello, D.; Rupp, T.; Dauzat, M.; Obert, P.; Nottin, S. Endurance Training Minimizes Age-Related Changes of Left Ventricular Twist-Untwist Mechanics. J. Am. Soc. Echocardiogr. 2014, 27, 1208–1215. [Google Scholar] [CrossRef]
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia Nervosa and Heart Disease: A Systematic Review. Eat. Weight Disord. EWD 2019, 24, 199–207. [Google Scholar] [CrossRef]
- Mazurak, N.; Enck, P.; Muth, E.; Teufel, M.; Zipfel, S. Heart Rate Variability as a Measure of Cardiac Autonomic Function in Anorexia Nervosa: A Review of the Literature. Eur. Eat. Disord. Rev. 2011, 19, 87–99. [Google Scholar] [CrossRef]
- Murialdo, G.; Casu, M.; Falchero, M.; Brugnolo, A.; Patrone, V.; Cerro, P.F.; Ameri, P.; Andraghetti, G.; Briatore, L.; Copello, F.; et al. Alterations in the Autonomic Control of Heart Rate Variability in Patients with Anorexia or Bulimia Nervosa: Correlations between Sympathovagal Activity, Clinical Features, and Leptin Levels. J. Endocrinol. Invest. 2007, 30, 356–362. [Google Scholar] [CrossRef]
- Vigo, D.E.; Castro, M.N.; Dörpinghaus, A.; Weidema, H.; Cardinali, D.P.; Siri, L.N.; Rovira, B.; Fahrer, R.D.; Nogués, M.; Leiguarda, R.C.; et al. Nonlinear Analysis of Heart Rate Variability in Patients with Eating Disorders. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2008, 9, 183–189. [Google Scholar] [CrossRef]
- Decuypere, E.; Van As, P.; Van der Geyten, S.; Darras, V.M. Thyroid Hormone Availability and Activity in Avian Species: A Review. Domest. Anim. Endocrinol. 2005, 29, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.M.; Yoshiuchi, K.; Kumano, H.; Sasaki, T.; Kuboki, T. Changes in Heart Rate with Refeeding in Anorexia Nervosa: A Pilot Study. J. Psychosom. Res. 2006, 61, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, C.; Tudoran, M.; Vlad, M.; Balas, M.; Ciocarlie, T.; Parv, F. Alterations of Heart Rate Variability and Turbulence in Female Patients with Hyperthyroidism of Various Severities. Niger. J. Clin. Pract. 2019, 22, 1349–1355. [Google Scholar] [CrossRef]
- Vázquez, M.; Olivares, J.L.; Fleta, J.; Lacambra, I.; González, M. Cardiac Disorders in Young Women with Anorexia Nervosa. Rev. Esp. Cardiol. Engl. Ed. 2003, 56, 669–673. [Google Scholar]
- Masson, S.; Latini, R.; Anand, I.S.; Vago, T.; Angelici, L.; Barlera, S.; Missov, E.D.; Clerico, A.; Tognoni, G.; Cohn, J.N.; et al. Direct Comparison of B-Type Natriuretic Peptide (BNP) and Amino-Terminal ProBNP in a Large Population of Patients with Chronic and Symptomatic Heart Failure: The Valsartan Heart Failure (Val-HeFT) Data. Clin. Chem. 2006, 52, 1528–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zastrow, A.; Wolf, J.; Giannitsis, E.; Katus, H.; Herzog, W.; Friederich, H.-C.; Mussler, C. Elevated Myocardial Enzymes and Natriuretic Peptides in Anorexia Nervosa: Prototypic Condition for the Pathophysiology of Cachexia? Cardiology 2011, 118, 256–259. [Google Scholar] [CrossRef]
- Kalra, P.R.; Tigas, S. Regulation of Lipolysis: Natriuretic Peptides and the Development of Cachexia. Int. J. Cardiol. 2002, 85, 125–132. [Google Scholar] [CrossRef]
- Boettler, P.; Hartmann, M.; Watzl, K.; Maroula, E.; Schultemoenting, J.; Knirsch, W.; Dittrich, S.; Kececioglu, D. Heart Rate Effects on Strain and Strain Rate in Healthy Children. J. Am. Soc. Echocardiogr. 2005, 18, 1121–1130. [Google Scholar] [CrossRef]





| Variables | AN (n = 26) | Controls (n = 33) | |
|---|---|---|---|
| without WR (n = 16) | with WR (n = 10) | ||
| Age (years) | 13.9 ± 1.6 | 15.7 ± 1.9 #,* | 14.1 ± 2.0 |
| Anthropometry | |||
| Height (cm) | 159.9 ± 10.3 | 159.5 ± 7.4 | 162.6 ± 10.0 |
| Body mass (kg) | 39.0 ± 6.9 *** | 43.5 ± 9.6 * | 51.2 ± 9.8 |
| BMI (kg.m−2) | 15.2 ± 1.7 *** | 16.9 ± 2.2 #,** | 19.2 ± 2.3 |
| BSA (m2) | 1.30 ± 1.15 *** | 1.38 ± 0.19 * | 1.52 ± 0.19 |
| Bioimpedance analysis | |||
| AFT (mm) | 7.4 ± 3.7 ** | 10.0 ± 3.9 | 14.1 ± 7.0 |
| Body fat mass (%) | 9.8 ± 5.5 *** | 15.0 ± 6.1 #,* | 20.0 ± 6.6 |
| Lean body mass (%) | 86.9 ± 5.9 *** | 81.8 ± 5.9 #,* | 76.6 ± 6.4 |
| Hemodynamic constants | |||
| Heart rate (bpm) | 55 ± 7 ###,*** | 65 ± 7 | 68 ± 6 |
| Systolic BP (mmHg) | 98 ± 16 *** | 100 ± 10 *** | 110 ± 8 |
| Diastolic BP (mmHg) | 65 ± 13 | 61 ± 7 | 67 ± 7 |
| Mean BP (mmHg) | 76 ± 14 * | 74 ± 7 * | 81 ± 7 |
| Variables | AN (n = 26) | Controls (n = 33) | |
|---|---|---|---|
| without WR (n = 16) | with WR (n = 10) | ||
| LV morphology | |||
| LV septum thickness (cm) | 0.71 ± 0.13 | 0.72 ± 0.19 | 0.77 ± 0.12 |
| LV posterior wall thickness (cm) | 0.63 ± 0.09 ** | 0.73 ± 0.15 # | 0.75 ± 0.12 |
| LV end-diastolic volume (mL) | 78 ± 20 | 81 ± 19 | 86 ± 18 |
| LV end-systolic volume (mL) | 27 ± 8 | 32 ± 8 | 31 ± 7 |
| LVM (g) | 74 ± 18 ** | 88 ± 36 | 96 ± 24 |
| LVM2.7 (g.m−2.7) | 21 ± 5 ** | 24 ± 7 | 26 ± 5 |
| LV function | |||
| Standard parameters | |||
| E wave (cm.s−1) | 85 ± 18 | 82 ± 15 | 82 ± 14 |
| A wave (cm.s−1) | 30 ± 7 *** | 32 ± 5 ** | 41 ± 7 |
| E/A | 3.0 ± 1.0 *** | 2.7 ± 0.7 ** | 2.0 ± 0.5 |
| Stroke volume index (mL.m−2) | 38.1 ± 6.4 | 35.0 ± 4.7 | 35.6 ± 5.1 |
| CO index (L.min−1.m−2) | 1.9 ± 0.3 *** | 2.1 ± 0.5 * | 2.5 ± 0.4 |
| Ejection fraction (%) | 65 ± 4 | 61 ± 5 | 64 ± 6 |
| Tissue Doppler imaging parameters | |||
| E’ (cm.s−1) | 14.1 ± 1.1 | 14.6 ± 2.4 | 15.0 ± 1.5 |
| A’ (cm.s−1) | 4.8 ± 0.7 *** | 5.6 ± 1.0 | 6.5 ± 1.2 |
| E’/A’ | 3.0 ± 0.5 *** | 2.6 ± 0.4 | 2.4 ± 0.4 |
| E/E’ lat | 5.3 ± 1.1 | 4.8 ± 0.7 | 4.7 ± 0.8 |
| S’ (cm.s−1) | 8.1 ± 0.8 ** | 8.7 ± 1.2 | 9.1 ± 1.0 |
| Speckle tracking echocardiography parameters | |||
| GLS (%) | −19.1 ± 1.8 ** | −18.4 ± 2.3 | −16.9 ± 2.8 |
| Model | Variables | B (Standardized Coefficient) | R2 | p |
|---|---|---|---|---|
| Relationship between SD1/SD2 and LVM 2.7, body fat mass, T3, NT-proBNP | ||||
| 1 | T3 | −0.651 | 0.424 | <0.001 |
| Relationship between LVM2.7 and body fat mass, EFT, SBP, T3, NT-proBNP | ||||
| 1 | Body fat mass | 0.636 | 0.404 | <0.001 |
| 2 | Body fat mass Systolic blood pressures | 0.443 0.368 | 0.503 | <0.001 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paysal, J.; Thireau, J.; Terral, D.; Rochette, E.; Obert, P.; Merlin, E.; Nottin, S. Cardiac Remodeling and Its Determinants in Anorexia Nervosa Adolescents: Impact of Weight Recovery. Children 2022, 9, 458. https://doi.org/10.3390/children9040458
Paysal J, Thireau J, Terral D, Rochette E, Obert P, Merlin E, Nottin S. Cardiac Remodeling and Its Determinants in Anorexia Nervosa Adolescents: Impact of Weight Recovery. Children. 2022; 9(4):458. https://doi.org/10.3390/children9040458
Chicago/Turabian StylePaysal, Justine, Jérôme Thireau, Daniel Terral, Emmanuelle Rochette, Philippe Obert, Etienne Merlin, and Stéphane Nottin. 2022. "Cardiac Remodeling and Its Determinants in Anorexia Nervosa Adolescents: Impact of Weight Recovery" Children 9, no. 4: 458. https://doi.org/10.3390/children9040458