Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review
Abstract
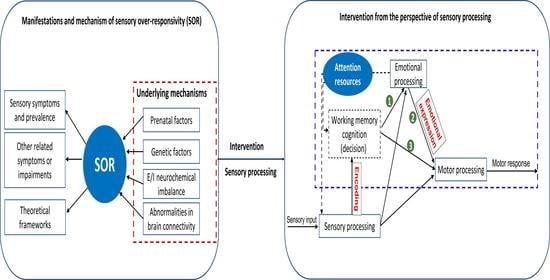
:1. Introduction
2. Method
2.1. Aims of the Current Review
2.2. Search Strategy
2.3. Study Selection
3. Result
3.1. Interventions for SOR in People with ASD
3.2. Physical Activity (PA)
3.3. Ayres Sensory Integration Therapy (SIT)
3.4. Mindfulness-Based Training (MBCT)
3.5. Cognitive Behavioral Therapy (CBT)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425558. [Google Scholar]
- Ben-Sasson, A.; Carter, A.S.; Briggs-Gowan, M.J. The development of sensory over-responsivity from infancy to elementary school. J. Abnorm. Child Psychol. 2010, 38, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Carter, A.S.; Briggs-Gowan, M.J. Sensory over-responsivity in elementary school: Prevalence and social-emotional correlates. J. Abnorm. Child Psychol. 2009, 37, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Gal, E.; Fluss, R.; Katz-Zetler, N.; Cermak, S.A. Update of a Meta-analysis of Sensory Symptoms in ASD: A New Decade of Research. J. Autism Dev. Disord. 2019, 49, 4974–4996. [Google Scholar] [CrossRef]
- DuBois, D.; Lymer, E.; Gibson, B.E.; Desarkar, P.; Nalder, E. Assessing Sensory Processing Dysfunction in Adults and Adolescents with Autism Spectrum Disorder: A Scoping Review. Brain Sci. 2017, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Baron-Cohen, S.; Ashwin, E.; Ashwin, C.; Tavassoli, T.; Chakrabarti, B. Talent in autism: Hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, A.; Crewther, D.P. Magnocellular visual evoked potential delay with high autism spectrum quotient yields a neural mechanism for altered perception. Brain 2010, 133, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Blake, R.; Turner, L.M.; Smoski, M.J.; Pozdol, S.L.; Stone, W.L. Visual recognition of biological motion is impaired in children with autism. Psychol. Sci. 2003, 14, 151–157. [Google Scholar] [CrossRef]
- Joseph, R.M.; Keehn, B.; Connolly, C.; Wolfe, J.M.; Horowitz, T.S. Why is visual search superior in autism spectrum disorder? Dev. Sci. 2009, 12, 1083–1096. [Google Scholar] [CrossRef]
- Robertson, C.E.; Kravitz, D.J.; Freyberg, J.; Baron-Cohen, S.; Baker, C.I. Tunnel vision: Sharper gradient of spatial attention in autism. J. Neurosci. 2013, 33, 6776–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.J. Sensational Kids: Hope and Help for Children with Sensory Processing Disorder (SPD); Penguin: London, UK, 2014; ISBN 039916782X. [Google Scholar]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M.; Baron-Cohen, S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and attention abnormalities in autistic spectrum disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, W. The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants Young Child. 1997, 9, 23–35. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Cermak, S.A.; Orsmond, G.I.; Tager-Flusberg, H.; Carter, A.S.; Kadlec, M.B.; Dunn, W. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. Am. J. Occup. Ther. 2007, 61, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, L.; Goddard, L.; Pring, L. Sensory processing in adults with autism spectrum disorders. Autism 2009, 13, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Andrés, M.I.; Sanz-Cerverza, P.; Salgado-Burgos, C.; Tárraga-Mínguez, R.; Pastor-Cerezuela, G. Comparative study of sensory modulation vulnerabilities in children with and without ASD in family and school contexts. J. Occup. Ther. Sch. Early Interv. 2018, 11, 318–328. [Google Scholar] [CrossRef]
- Dunn, W. Supporting Children to Participate Successfully in Everyday Life by Using Sensory Processing Knowledge. Infants Young Child. 2007, 20, 84–101. [Google Scholar] [CrossRef] [Green Version]
- Keuler, M.M.; Schmidt, N.L.; van Hulle, C.A.; Lemery-Chalfant, K.; Goldsmith, H.H. Sensory overresponsivity: Prenatal risk factors and temperamental contributions. J. Dev. Behav. Pediatr. 2011, 32, 533–541. [Google Scholar] [CrossRef]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Smith Roley, S.; Schaaf, R.C. Neural Foundations of Ayres Sensory Integration®. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, S.; Lane, S.J. Diagnostic validity of sensory over-responsivity: A review of the literature and case reports. J. Autism. Dev. Disord. 2008, 38, 516–529. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Working Together for Health: The World Health Report 2006/World Health Organization; World Health Organization: Geneva, Switzerland, 2006; ISBN 9789241563178. [Google Scholar]
- Carter, A.S.; Ben-Sasson, A.; Briggs-Gowan, M.J. Sensory over-responsivity, psychopathology, and family impairment in school-aged children. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, M.O.; Petroski, G.F. Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Med. 2015, 16, 270–279. [Google Scholar] [CrossRef]
- Carpenter, K.L.H.; Baranek, G.T.; Copeland, W.E.; Compton, S.; Zucker, N.; Dawson, G.; Egger, H.L. Sensory Over-Responsivity: An Early Risk Factor for Anxiety and Behavioral Challenges in Young Children. J. Abnorm. Child Psychol. 2019, 47, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- van Hulle, C.A.; Esbensen, K.; Goldsmith, H.H. Co-occurrence of Sensory Overresponsivity with Obsessive-Compulsive Symptoms in Childhood and Early Adolescence. J. Dev. Behav. Pediatr. 2019, 40, 377–382. [Google Scholar] [CrossRef]
- Podoly, T.Y.; Derby, D.S.; Ben-Sasson, A. Sensory over-responsivity and obsessive-compulsive disorder: Measuring habituation and sensitivity through self-report, physiological and behavioral indices. J. Psychiatr. Res. 2022, 149, 266–273. [Google Scholar] [CrossRef]
- Greenspan, S.I.; Wieder, S. Diagnostic Classification in Infancy and Early Childhood. In Psychiatry; Tasman, A., Kay, J., Lieberman, J.A., First, M.B., Maj, M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 679–688. ISBN 9780470515167. [Google Scholar]
- James, K.; Miller, L.J.; Schaaf, R.; Nielsen, D.M.; Schoen, S.A. Phenotypes within sensory modulation dysfunction. Compr. Psychiatry 2011, 52, 715–724. [Google Scholar] [CrossRef]
- Horder, J.; Wilson, C.E.; Mendez, M.A.; Murphy, D.G. Autistic traits and abnormal sensory experiences in adults. J. Autism Dev. Disord. 2014, 44, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Vasa, R.A.; Keefer, A.; McDonald, R.G.; Hunsche, M.C.; Kerns, C.M. A Scoping Review of Anxiety in Young Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 2038–2057. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Rodgers, J.; McConachie, H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. J. Autism. Dev. Disord. 2009, 39, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, R.L.; Agnew, J.A.; Miller, L.J.; Gralla, J.; Pan, Z.; Goldson, E.; Ledbetter, J.C.; Dinkins, J.P.; Hooks, E. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Res. Autism Spectr. Disord. 2008, 2, 660–670. [Google Scholar] [CrossRef]
- Boyd, B.A.; McBee, M.; Holtzclaw, T.; Baranek, G.T.; Bodfish, J.W. Relationships among Repetitive Behaviors, Sensory Features, and Executive Functions in High Functioning Autism. Res. Autism Spectr. Disord. 2009, 3, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Sasson, A.; Cermak, S.A.; Orsmond, G.I.; Tager-Flusberg, H.; Kadlec, M.B.; Carter, A.S. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. J. Child Psychol. Psychiatry 2008, 49, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Glod, M.; Riby, D.M.; Honey, E.; Rodgers, J. Psychological Correlates of Sensory Processing Patterns in Individuals with Autism Spectrum Disorder: A Systematic Review. Rev J Autism Dev Disord 2015, 2, 199–221. [Google Scholar] [CrossRef] [Green Version]
- Green, S.A.; Ben-Sasson, A.; Soto, T.W.; Carter, A.S. Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. J. Autism Dev. Disord. 2012, 42, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Lane, S.J.; Reynolds, S.; Dumenci, L. Sensory overresponsivity and anxiety in typically developing children and children with autism and attention deficit hyperactivity disorder: Cause or coexistence? Am. J. Occup. Ther. 2012, 66, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Dellapiazza, F.; Vernhet, C.; Blanc, N.; Miot, S.; Schmidt, R.; Baghdadli, A. Links between sensory processing, adaptive behaviours, and attention in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2018, 270, 78–88. [Google Scholar] [CrossRef]
- Boyd, B.A.; Baranek, G.T.; Sideris, J.; Poe, M.D.; Watson, L.R.; Patten, E.; Miller, H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Res. 2010, 3, 78–87. [Google Scholar] [CrossRef]
- Muskett, A.; Capriola-Hall, N.N.; Radtke, S.R.; Factor, R.; Scarpa, A. Repetitive behaviors in Autism Spectrum Disorder: Associations with depression and anxiety symptoms. Res. Autism Spectr. Disord. 2019, 68, 101449. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Vigod, S.; Pullenayegum, E.; Kerns, C.M.; Mirenda, P.; Smith, I.M.; Vaillancourt, T.; Volden, J.; Waddell, C.; Zwaigenbaum, L.; et al. Repetitive Behavior Severity as an Early Indicator of Risk for Elevated Anxiety Symptoms in Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 890–899.e3. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-H.; Fu, C.-P.; Cermak, S.A.; Lu, L.; Shieh, J.-Y. Emotional and behavioral problems in preschool children with autism: Relationship with sensory processing dysfunction. Res. Autism Spectr. Disord. 2011, 5, 1441–1450. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Huang, P.-C.; Wang, T.-Y.; Chang, Y.-C.; Lin, L.-Y. Relationship Among Sensory Over-Responsivity, Problem Behaviors, and Anxiety in Emerging Adults with Autism Spectrum Disorder. Neuropsychiatr. Dis. Treat. 2020, 16, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.M.; Nevill, R.E.; Mazurek, M.O. Sensory over-responsivity, repetitive behavior, and emotional functioning in boys with and without autism spectrum disorder. Res. Autism Spectr. Disord. 2020, 75, 101573. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chan, P.-Y.S.; Hsieh, Y.-W.; Chen, K.-F. A meta-analysis of mismatch negativity in children with attention deficit-hyperactivity disorders. Neurosci. Lett. 2016, 612, 132–137. [Google Scholar] [CrossRef]
- Oliver, C.; Adams, D.; Allen, D.; Crawford, H.; Heald, M.; Moss, J.; Richards, C.; Waite, J.; Welham, A.; Wilde, L.; et al. The behaviour and wellbeing of children and adults with severe intellectual disability and complex needs: The Be-Well checklist for carers and professionals. Paediatr. Child Health 2020, 30, 416–424. [Google Scholar] [CrossRef]
- Lewin, A.B.; Wu, M.S.; Murphy, T.K.; Storch, E.A. Sensory Over-Responsivity in Pediatric Obsessive Compulsive Disorder. J. Psychopathol. Behav. Assess 2015, 37, 134–143. [Google Scholar] [CrossRef]
- Conelea, C.A.; Carter, A.C.; Freeman, J.B. Sensory over-responsivity in a sample of children seeking treatment for anxiety. J. Dev. Behav. Pediatr. 2014, 35, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.L.; Campi, E.; Baranek, G.T. Associations among sensory hyperresponsiveness, restricted and repetitive behaviors, and anxiety in autism: An integrated systematic review. Res. Autism Spectr. Disord. 2021, 83, 101763. [Google Scholar] [CrossRef]
- Narzisi, A.; Fabbri-Destro, M.; Crifaci, G.; Scatigna, S.; Maugeri, F.; Berloffa, S.; Fantozzi, P.; Prato, A.; Muccio, R.; Valente, E.; et al. Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2). J. Clin. Med. 2022, 11, 1668. [Google Scholar] [CrossRef]
- Rogers, S.J.; Hepburn, S.; Wehner, E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J. Autism Dev. Disord. 2003, 33, 631–642. [Google Scholar] [CrossRef] [PubMed]
- White, S.W.; Oswald, D.; Ollendick, T.; Scahill, L. Anxiety in children and adolescents with autism spectrum disorders. Clin. Psychol. Rev. 2009, 29, 216–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, J.; Hederos Eriksson, L.; Hammarsten, R.; Lundin Remnélius, K.; Tillmann, J.; Isaksson, J.; Bölte, S. The impact of atypical sensory processing on adaptive functioning within and beyond autism: The role of familial factors. Autism 2021, 25, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Dovgan, K.; Neumeyer, A.M.; Malow, B.A. Course and Predictors of Sleep and Co-occurring Problems in Children with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2019, 49, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, A.; Soto, T.W.; Martínez-Pedraza, F.; Carter, A.S. Early sensory over-responsivity in toddlers with autism spectrum disorders as a predictor of family impairment and parenting stress. J. Child Psychol. Psychiatry 2013, 54, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Cascio, C.J.; Woynaroski, T.; Baranek, G.T.; Wallace, M.T. Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Res. 2016, 9, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Burns, C.O.; Dixon, D.R.; Novack, M.; Granpeesheh, D. A Systematic Review of Assessments for Sensory Processing Abnormalities in Autism Spectrum Disorder. Rev. J. Autism Dev. Disord. 2017, 4, 209–224. [Google Scholar] [CrossRef]
- Dickie, V.A.; Baranek, G.T.; Schultz, B.; Watson, L.R.; McComish, C.S. Parent reports of sensory experiences of preschool children with and without autism: A qualitative study. Am. J. Occup. Ther. 2009, 63, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli, T.; Hoekstra, R.A.; Baron-Cohen, S. The Sensory Perception Quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Mol. Autism 2014, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Kern, J.K.; Trivedi, M.H.; Grannemann, B.D.; Garver, C.R.; Johnson, D.G.; Andrews, A.A.; Savla, J.S.; Mehta, J.A.; Schroeder, J.L. Sensory correlations in autism. Autism 2007, 11, 123–134. [Google Scholar] [CrossRef]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Talay-Ongan, A.; Wood, K. Unusual Sensory Sensitivities in Autism: A possible crossroads. Int. J. Disabil. Dev. Educ. 2000, 47, 201–212. [Google Scholar] [CrossRef]
- McCormick, C.; Hepburn, S.; Young, G.S.; Rogers, S.J. Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: A longitudinal study. Autism 2016, 20, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramappa, S.; Anderson, A.; Jung, J.; Chu, R.; Cummings, K.K.; Patterson, G.; Okada, N.J.; Green, S.A. An Observed Assessment of Sensory Responsivity in Autism Spectrum Disorders: Associations with Diagnosis, Age, and Parent Report. J. Autism Dev. Disord. 2022, 1–13. [Google Scholar] [CrossRef]
- Happé, F. The Weak Central Coherence Account of Autism. In Handbook of Autism and Pervasive Developmental Disorders; Volkmar, F.R., Paul, R., Klin, A., Cohen, D., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 640–649. ISBN 9780471716969. [Google Scholar]
- Brock, J.; Brown, C.C.; Boucher, J.; Rippon, G. The temporal binding deficit hypothesis of autism. Dev. Psychopathol. 2002, 14, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Shultz, S.; Hudac, C.M.; Vander Wyk, B.C. Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry 2011, 52, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Richardson, H.; Gweon, H.; Dodell-Feder, D.; Malloy, C.; Pelton, H.; Keil, B.; Kanwisher, N.; Saxe, R. Response patterns in the developing social brain are organized by social and emotion features and disrupted in children diagnosed with autism spectrum disorder. Cortex 2020, 125, 12–29. [Google Scholar] [CrossRef] [Green Version]
- Case-Smith, J.; Butcher, L.; Reed, D. Parents’ report of sensory responsiveness and temperament in preterm infants. Am. J. Occup. Ther. 1998, 52, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, H.H.; van Hulle, C.A.; Arneson, C.L.; Schreiber, J.E.; Gernsbacher, M.A. A population-based twin study of parentally reported tactile and auditory defensiveness in young children. J. Abnorm. Child Psychol. 2006, 34, 393–407. [Google Scholar] [CrossRef]
- Schneider, M.L.; Moore, C.F.; Gajewski, L.L.; Larson, J.A.; Roberts, A.D.; Converse, A.K.; DeJesus, O.T. Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008, 79, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.L.; Moore, C.F.; Adkins, M.M. The effects of prenatal alcohol exposure on behavior: Rodent and primate studies. Neuropsychol. Rev. 2011, 21, 186–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C. Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules 2015, 5, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.I.; Donkers, F.C.L.; Winkler, I.; Otte, R.A.; van den Bergh, B.R.H. Maternal mindfulness and anxiety during pregnancy affect infants’ neural responses to sounds. Soc. Cogn. Affect. Neurosci. 2015, 10, 453–460. [Google Scholar] [CrossRef] [Green Version]
- van Hulle, C.A.; Schmidt, N.L.; Goldsmith, H.H. Is sensory over-responsivity distinguishable from childhood behavior problems? A phenotypic and genetic analysis. J. Child Psychol. Psychiatry 2012, 53, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.L.; van Hulle, C.A.; Brooker, R.J.; Meyer, L.R.; Lemery-Chalfant, K.; Goldsmith, H.H. Wisconsin Twin Research: Early development, childhood psychopathology, autism, and sensory over-responsivity. Twin Res. Hum. Genet. 2013, 16, 376–384. [Google Scholar] [CrossRef]
- Glod, M.; Riby, D.M.; Honey, E.; Rodgers, J. Sensory atypicalities in dyads of children with autism spectrum disorder (ASD) and their parents. Autism Res. 2017, 10, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Uljarević, M.; Prior, M.R.; Leekam, S.R. First evidence of sensory atypicality in mothers of children with Autism Spectrum Disorder (ASD). Mol. Autism 2014, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef] [Green Version]
- DeLorey, T.M.; Sahbaie, P.; Hashemi, E.; Li, W.-W.; Salehi, A.; Clark, D.J. Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behav. Brain Res. 2011, 216, 36–45. [Google Scholar] [CrossRef]
- Coghlan, S.; Horder, J.; Inkster, B.; Mendez, M.A.; Murphy, D.G.; Nutt, D.J. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci. Biobehav. Rev. 2012, 36, 2044–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oblak, A.; Gibbs, T.T.; Blatt, G.J. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009, 2, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Burnashev, N.; Szepetowski, P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr. Opin. Pharmacol. 2015, 20, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Wilson, L.B. γ-band abnormalities as markers of autism spectrum disorders. Biomark. Med. 2014, 8, 353–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Wood, E.T.; Cummings, K.K.; Jung, J.; Patterson, G.; Okada, N.; Guo, J.; O’Neill, J.; Dapretto, M.; Bookheimer, S.Y.; Green, S.A. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl. Psychiatry 2021, 11, 39. [Google Scholar] [CrossRef]
- Cardon, G.J.; Hepburn, S.; Rojas, D.C. Structural Covariance of Sensory Networks, the Cerebellum, and Amygdala in Autism Spectrum Disorder. Front. Neurol. 2017, 8, 615. [Google Scholar] [CrossRef] [Green Version]
- Kleinhans, N.M.; Müller, R.-A.; Cohen, D.N.; Courchesne, E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008, 1221, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Green, S.A.; Hernandez, L.M.; Bowman, H.C.; Bookheimer, S.Y.; Dapretto, M. Sensory over-responsivity and social cognition in ASD: Effects of aversive sensory stimuli and attentional modulation on neural responses to social cues. Dev. Cogn. Neurosci. 2018, 29, 127–139. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Sandbank, M.; Bottema-Beutel, K.; Crowley, S.; Cassidy, M.; Dunham, K.; Feldman, J.I.; Crank, J.; Albarran, S.A.; Raj, S.; Mahbub, P.; et al. Project AIM: Autism intervention meta-analysis for studies of young children. Psychol. Bull. 2020, 146, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bass, M.M.; Duchowny, C.A.; Llabre, M.M. The effect of therapeutic horseback riding on social functioning in children with autism. J. Autism Dev. Disord. 2009, 39, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Fazlioğlu, Y.; Baran, G. A sensory integration therapy program on sensory problems for children with autism. Percept. Mot. Ski. 2008, 106, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kashefimehr, B.; Kayihan, H.; Huri, M. The Effect of Sensory Integration Therapy on Occupational Performance in Children With Autism. OTJR Thorofare N. J. 2018, 38, 75–83. [Google Scholar] [CrossRef]
- Pfeiffer, B.A.; Koenig, K.; Kinnealey, M.; Sheppard, M.; Henderson, L. Effectiveness of sensory integration interventions in children with autism spectrum disorders: A pilot study. Am. J. Occup. Ther. 2011, 65, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, K.; Moulding, R. Sensory-Processing Sensitivity, dispositional mindfulness and negative psychological symptoms. Personal. Individ. Differ. 2012, 53, 341–346. [Google Scholar] [CrossRef]
- Edgington, L.; Hill, V.; Pellicano, E. The design and implementation of a CBT-based intervention for sensory processing difficulties in adolescents on the autism spectrum. Res. Dev. Disabil. 2016, 59, 221–233. [Google Scholar] [CrossRef]
- Stavropoulos, K.K.-M. Using neuroscience as an outcome measure for behavioral interventions in Autism spectrum disorders (ASD): A review. Res. Autism Spectr. Disord. 2017, 35, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.; Veytsman, E.; Martin, A.M.; Blacher, J.; Stavropoulos, K.K.M. Increased Neural Reward Responsivity in Adolescents with ASD after Social Skills Intervention. Brain Sci. 2020, 10, 402. [Google Scholar] [CrossRef]
- Wickens, C.D.; Carswell, C.M. Information Processing. In Handbook of Human Factors and Ergonomics; Salvendy, G., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 117–161. ISBN 9780470528389. [Google Scholar]
- Funahashi, S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Zalla, T.; Sperduti, M. The amygdala and the relevance detection theory of autism: An evolutionary perspective. Front. Hum. Neurosci. 2013, 7, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristell, M.; Hutchinson, K.M.; Alessio, H.M. Effects of exercise training on hearing ability. Scand. Audiol. 1998, 27, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, E.; Aziz-Zadeh, L.; Cermak, S. Ayres Theories of Autism and Sensory Integration Revisited: What Contemporary Neuroscience Has to Say. Brain Sci. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Stubbs, B.; Zou, W.; Zheng, W.; Lu, W.; Gao, Y.; Chen, K.; Wang, S.; Liu, J.; Huang, Y.; et al. Aerobic exercise impacts the anterior cingulate cortex in adolescents with subthreshold mood syndromes: A randomized controlled trial study. Transl. Psychiatry 2020, 10, 155. [Google Scholar] [CrossRef]
- Yano, K.; Oishi, K. The relationships among daily exercise, sensory-processing sensitivity, and depressive tendency in Japanese university students. Personal. Individ. Differ. 2018, 127, 49–53. [Google Scholar] [CrossRef]
- Watling, R.; Hauer, S. Effectiveness of Ayres Sensory Integration® and Sensory-Based Interventions for People With Autism Spectrum Disorder: A Systematic Review. Am. J. Occup. Ther. 2015, 69, 6905180030p1–6905180030p12. [Google Scholar] [CrossRef] [Green Version]
- Ayres, A.J.; Tickle, L.S. Hyper-responsivity to touch and vestibular stimuli as a predictor of positive response to sensory integration procedures by autistic children. Am. J. Occup. Ther. 1980, 34, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Baranek, G.T. Efficacy of sensory and motor interventions for children with autism. J Autism Dev Disord 2002, 32, 397–422. [Google Scholar] [CrossRef]
- Ayres, A.J. Tactile functions. Their relation to hyperactive and perceptual motor behavior. Am. J. Occup. Ther. 1964, 18, 6–11. [Google Scholar]
- Ayres, A.J. Interrelationships among perceptual-motor functions in children. Am. J. Occup. Ther. 1966, 20, 68–71. [Google Scholar]
- Wyller, H.B.; Wyller, V.B.B.; Crane, C.; Gjelsvik, B. The relationship between sensory processing sensitivity and psychological distress: A model of underpinning mechanisms and an analysis of therapeutic possibilities. Scand. Psychol. 2017, 4, e15. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life, Kindle ed.; Hyperion e-book: New York, NY, UK, 2009; ISBN 9781401394677. [Google Scholar]
- Kuyken, W.; Watkins, E.; Holden, E.; White, K.; Taylor, R.S.; Byford, S.; Evans, A.; Radford, S.; Teasdale, J.D.; Dalgleish, T. How does mindfulness-based cognitive therapy work? Behav. Res. Ther. 2010, 48, 1105–1112. [Google Scholar] [CrossRef]
- Khoury, B.; Lecomte, T.; Fortin, G.; Masse, M.; Therien, P.; Bouchard, V.; Chapleau, M.-A.; Paquin, K.; Hofmann, S.G. Mindfulness-based therapy: A comprehensive meta-analysis. Clin. Psychol. Rev. 2013, 33, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kuyken, W.; Warren, F.C.; Taylor, R.S.; Whalley, B.; Crane, C.; Bondolfi, G.; Hayes, R.; Huijbers, M.; Ma, H.; Schweizer, S.; et al. Efficacy of Mindfulness-Based Cognitive Therapy in Prevention of Depressive Relapse: An Individual Patient Data Meta-analysis From Randomized Trials. JAMA Psychiatry 2016, 73, 565–574. [Google Scholar] [CrossRef] [PubMed]
- MacLean, K.A.; Ferrer, E.; Aichele, S.R.; Bridwell, D.A.; Zanesco, A.P.; Jacobs, T.L.; King, B.G.; Rosenberg, E.L.; Sahdra, B.K.; Shaver, P.R.; et al. Intensive meditation training improves perceptual discrimination and sustained attention. Psychol. Sci. 2010, 21, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.; Malinowski, P. Meditation, mindfulness and cognitive flexibility. Conscious. Cogn. 2009, 18, 176–186. [Google Scholar] [CrossRef]
- Cachia, R.L.; Anderson, A.; Moore, D.W. Mindfulness in Individuals with Autism Spectrum Disorder: A Systematic Review and Narrative Analysis. Rev. J. Autism Dev. Disord. 2016, 3, 165–178. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Pospos, S.; Lavretsky, H. The Neural Mechanisms of Meditative Practices: Novel Approaches for Healthy Aging. Curr. Behav. Neurosci. Rep. 2016, 3, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, B.P.; Jagiellowicz, J.; Aron, E.; Marhenke, R.; Aron, A. Sensory processing sensitivity and childhood quality’s effects on neural responses to emotional stimuli. Clin. Neuropsychiatry J. Treat. Eval. 2017, 14, 359–373. [Google Scholar]
- Hofmann, S.G. An Introduction to Modern CBT: Psychological Solutions to Mental Health Problems; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1119951410. [Google Scholar]
- Maddox, B.B.; Miyazaki, Y.; White, S.W. Long-Term Effects of CBT on Social Impairment in Adolescents with ASD. J Autism Dev. Disord. 2017, 47, 3872–3882. [Google Scholar] [CrossRef]
- van Steensel, F.J.A.; Bögels, S.M. CBT for anxiety disorders in children with and without autism spectrum disorders. J. Consult. Clin. Psychol. 2015, 83, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Reaven, J.; Moody, E.J.; Grofer Klinger, L.; Keefer, A.; Duncan, A.; O’Kelley, S.; Meyer, A.; Hepburn, S.; Blakeley-Smith, A. Training clinicians to deliver group CBT to manage anxiety in youth with ASD: Results of a multisite trial. J. Consult. Clin. Psychol. 2018, 86, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Reyes, N.M. Improving emotion regulation with CBT in young children with high functioning autism spectrum disorders: A pilot study. Behav. Cogn. Psychother. 2011, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Keefer, A.; Kreiser, N.L.; Singh, V.; Blakeley-Smith, A.; Duncan, A.; Johnson, C.; Klinger, L.; Meyer, A.; Reaven, J.; Vasa, R.A. Intolerance of Uncertainty Predicts Anxiety Outcomes Following CBT in Youth with ASD. J. Autism Dev. Disord. 2017, 47, 3949–3958. [Google Scholar] [CrossRef]
- Reaven, J.; Meyer, A.T.; Pickard, K.; Boles, R.E.; Hayutin, L.; Middleton, C.; Reyes, N.M.; Hepburn, S.L.; Tanda, T.; Stahmer, A.; et al. Increasing Access and Reach: Implementing School-Based CBT for Anxiety in Students with ASD or Suspected ASD. Evid. -Based Pract. Child Adolesc. Ment. Health 2022, 7, 56–75. [Google Scholar] [CrossRef]
- Spain, D.; Blainey, S.H.; Vaillancourt, K. Group cognitive behaviour therapy (CBT) for social interaction anxiety in adults with autism spectrum disorders (ASD). Res. Autism Spectr. Disord. 2017, 41–42, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Muskett, A.; Radtke, S.; White, S.; Ollendick, T. Autism Spectrum Disorder and Specific Phobia: The Role of Sensory Sensitivity: Brief Review. Rev. J. Autism Dev. Disord. 2019, 6, 289–293. [Google Scholar] [CrossRef]
- Green, S.A.; Hernandez, L.; Tottenham, N.; Krasileva, K.; Bookheimer, S.Y.; Dapretto, M. Neurobiology of Sensory Overresponsivity in Youth With Autism Spectrum Disorders. JAMA Psychiatry 2015, 72, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Green, S.A.; Rudie, J.D.; Colich, N.L.; Wood, J.J.; Shirinyan, D.; Hernandez, L.; Tottenham, N.; Dapretto, M.; Bookheimer, S.Y. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 1158–1172. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.J.; Jassi, A.; Fullana, M.A.; Mack, H.; Johnston, K.; Heyman, I.; Murphy, D.G.; Mataix-Cols, D. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: A randomized controlled trial. Depress. Anxiety 2013, 30, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Sukhodolsky, D.G.; Bloch, M.H.; Panza, K.E.; Reichow, B. Cognitive-behavioral therapy for anxiety in children with high-functioning autism: A meta-analysis. Pediatrics 2013, 132, e1341–e1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, S.A.; Ben-Sasson, A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? J. Autism Dev. Disord. 2010, 40, 1495–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.Y.Y.; Yung, T.W.K.; Gomez, I.N.B.; Siu, A.M.H. Psychometric Properties of Sensory Processing and Self-Regulation Checklist (SPSRC). Occup. Ther. Int. 2019, 2019, 8796042. [Google Scholar] [CrossRef] [PubMed]


| Population Term | Sensory Term | Descriptor Term | Intervention Types |
|---|---|---|---|
| Sensory hyper-responsivity OR hyper-responsivity OR Over-responsivity OR hyper-reactivity | Autism OR autistic syndrome disorder OR autistic disorder OR autism spectrum disorder OR Asperger | Effective intervention OR training OR therapy | PA, SIT, MBCT, and CBT |
| Authors | Number/Age of Participants | Assessment | Intervention/Duration | Metrics of Sensory Alteration | Sensory Processing Results |
|---|---|---|---|---|---|
| Bass et al. (2009) [97] |
|
| PA: 1 h/wk; 12 weeks | The score of specific subscales of SP of sensory sensitivity was used to assess sensory alteration pre- and post-intervention | Experimental group demonstrated significantly improved social motivation and sensory sensitivity, as well as decreased inattention and distractibility |
| Fazlioğlu et al. (2008) [98] |
|
| SIT: 45 min/wk; 12 weeks | The scores of the evaluation forms were reassessed and compared between pre- and post-test | Sensory problems of children with autism improved, including hyper- and hypo-sensitivity to stimulation in experimental group |
| Kashefimehr et al. (2018) [99] |
|
| SIT: 1 h/wk; 12 weeks | The pretest and posttest SP scores were compared between the intervention | The intervention group showed significantly greater improvements in all factors and domains of SP |
| Pfeiffer et al. (2011) [100] |
|
| SIT: 45 min/wk; 6 wks | The pretest and posttest SPM scores were compared between the intervention | No significant differences in the scores on the SPM. A significant decrease in autistic mannerisms. |
| Bakker et al. (2012) [101] |
|
| MBCT: no intervention, only tested mindfulness awareness | In this research, we aimed to investigate the relationship between SPS, mindfulness, and distress using a cross-sectional methodology in a non-clinical sample | SPS was related to higher levels of depression, anxiety, and stress. Participants with low acceptance of mindfulness in particular had higher anxiety, while the SPS score of those with high acceptance of mindfulness had no significant relationship with anxiety. |
| Edgington et al. (2016) [102] |
|
| CBT: 45 min/wk; 8 wks | The pretest and posttest AASP scores were compared between the intervention | The intervention itself was feasible.No significant difference between pretest and posttest AASP scores.Adolescents with autism reported feeling better able to deal with sensory issues.Effectively raised meta-conscious awareness and self-regulation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.-L.; Lai, C.Y.Y.; Wong, M.N.K.; Kwong, T.C.; Choy, Y.S.; Mung, S.W.Y.; Chan, C.C.H. Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review. Children 2022, 9, 1584. https://doi.org/10.3390/children9101584
Yuan H-L, Lai CYY, Wong MNK, Kwong TC, Choy YS, Mung SWY, Chan CCH. Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review. Children. 2022; 9(10):1584. https://doi.org/10.3390/children9101584
Chicago/Turabian StyleYuan, Huan-Ling, Cynthia Y. Y. Lai, Mabel N. K. Wong, Tak Chun Kwong, Yat Sze Choy, Steve W. Y. Mung, and Chetwyn C. H. Chan. 2022. "Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review" Children 9, no. 10: 1584. https://doi.org/10.3390/children9101584