The Effect of Breastfeeding on Food Allergies in Newborns and Infants
Abstract
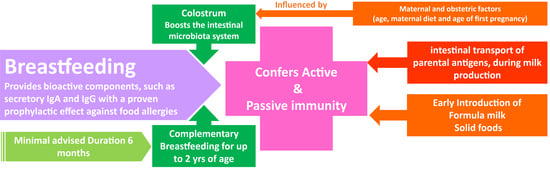
:1. Introduction
2. Breastfeeding and Allergies
3. Breastfeeding and Its Effects on Asthma and Atopic Dermatitis
4. The Effects of Breastfeeding on the Development of the Gut Microbiome
5. Colostrum and Allergies
6. Breastfeeding Duration and Effectiveness in Allergic Diseases
7. Introduction of Solid Foods and Food Allergies
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyce, J.A.; Assa′Ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Güngör, D.; Nadaud, P.; LaPergola, C.C.; Dreibelbis, C.; Wong, Y.P.; Terry, N.; Abrams, S.A.; Beker, L.; Jacobovits, T.; Järvinen, K.M.; et al. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: A systematic review. Am. J. Clin. Nutr. 2019, 109, 772S–799S. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Annals of allergy, asthma & immunology: Official publication of the American College of Allergy. Asthma Immunology. 2019, 123, 133–143. [Google Scholar]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Koukou, Z.; Theodoridou, A.; Taousani, E.; Antonakou, A.; Panteris, E.; Papadopoulou, S.-S.; Skordou, A.; Sifakis, S. Effectiveness of Non-Pharmacological Methods, Such as Breastfeeding, to Mitigate Pain in NICU Infants. Children 2022, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Andronikidi, P.E.; Eskitzis, P.; Iliadou, M.; Palaska, E.; Tzitiridou-Chatzopoulou, M.; Rigas, N.; Orovou, E. Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review. Viruses 2023, 15, 601. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Newborn Health: Guidelines Approved by the WHO Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2017.
- Hirata, N.; Kiuchi, M.; Pak, K.; Fukuda, R.; Mochimaru, N.; Mitsui, M.; Yoshida, K. Association between Maternal Characteristics and Immune Factors TGF-β1, TGF-β2, and IgA in Colostrum: An Exploratory Study in Japan. Nutrients 2022, 14, 3255. [Google Scholar] [CrossRef]
- Khaleva, E.; Gridneva, Z.; Geddes, D.T.; Oddy, W.H.; Colicino, S.; Blyuss, O.; Boyle, R.J.; Warner, J.O.; Munblit, D. Transforming growth factor beta in human milk and allergic outcomes in children: A systematic review. Clin. Exp. Allergy 2019, 49, 1201–1213. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, S.; Niggemann, B.; Arató, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow′s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Giannetti, A.; Vespasiani, G.T.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef]
- Protocol, A. ABM clinical protocol# 24: Allergic proctocolitis in the exclusively breastfed infant. Breastfeed. Med. 2011, 6, 435–440. [Google Scholar]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.I.; Pendower, U.; Santer, M.; Groetch, M.; Cohen, M.; Murch, S.H.; Williams, H.C.; Munblit, D.; Katz, Y.; Gupta, N.; et al. Detection and management of milk allergy: Delphi consensus study. Clin. Exp. Allergy 2022, 52, 848–858. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The not-so-sterile womb: Evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossny, E.M.; El-Ghoneimy, D.H.; El-Owaidy, R.H.; Mansour, M.G.; Hamza, M.T.; El-Said, A.F. Breast milk interleukin-7 and thymic gland development in infancy. Eur. J. Nutr. 2019, 59, 111–118. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Xypolias, D.Z.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef]
- Amazouz, H.; Roda, C.; Beydon, N.; Lezmi, G.; Bourgoin-Heck, M.; Just, J.; Momas, I.; Rancière, F. Mediterranean diet and lung function, sensitization, and asthma at school age: The PARIS cohort. Pediatr. Allergy Immunol. 2021, 32, 1437–1444. [Google Scholar] [CrossRef]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Azad, M.B.; Daley, D.; et al. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J. Pediatr. 2017, 190, 192–199.e2. [Google Scholar] [CrossRef]
- Ehlayel, M.S.; Bener, A. Duration of breast-feeding and the risk of childhood allergic diseases in a developing country. Allergy Asthma Proc. 2008, 29, 386–391. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Liao, S.L.; Su, K.W.; Tsai, M.H.; Hua, M.C.; Lai, S.H.; Chen, L.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Exclusive or partial breastfeeding for 6 months is associated with reduced milk sensitization and risk of eczema in early childhood: The PATCH Birth Cohort Study. Medicine 2016, 95, e3391. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Sicherer, S.; Greenhawt, M.; Campbell, D.; Chan, E.; Muraro, A.; Halken, S.; Katz, Y.; Ebisawa, M.; Eichenfield, L.; et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Pediatrics 2015, 136, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Martone, G.M.; Lehman, H.K.; Rideout, T.C.; Cameron, C.E.; Dashley, S.; Konnayil, B.J. Frequency of Infant Egg Consumption and Risk of Maternal-Reported Egg Allergy at 6 Years. J. Nutr. 2023, 153, 364–372. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [Green Version]
- Mathias, J.G.; Zhang, H.; Soto-Ramirez, N.; Karmaus, W. The association of infant feeding patterns with food allergy symptoms and food allergy in early childhood. Int. Breastfeed. J. 2019, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Grimshaw, K.; Logan, K.; O′Donovan, S.; Kiely, M.; Patient, K.; van Bilsen, J.; Beyer, K.; Campbell, D.E.; Garcia-Larsen, V.; Grabenhenrich, L.; et al. Modifying the infant′s diet to prevent food allergy. Arch. Dis. Child. 2016, 102, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.-K.; Liu, J.-L.; Chen, L.-J.; Li, J.-H.; Yang, J.-Z.; Xu, L.-L.; Chen, Y.-K.; Zhang, Q.-Y.; Li, X.-W.; Liu, Y.; et al. Gut microbiota mediates methamphetamine-induced hepatic inflammation via the impairment of bile acid homeostasis. Food Chem. Toxicol. 2022, 166, 113208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.; Zhao, J.; Wei, Y.; Zhai, S.; Tan, M.; Guan, K.; Huang, Z.; Chen, C. Lonicera rupicola Hook.f.et Thoms flavonoids ameliorated dysregulated inflammatory responses, intestinal barrier, and gut microbiome in ulcerative colitis via PI3K/AKT pathway. Phytomedicine 2022, 104, 154284. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, M.; Zheng, P.; Liu, R.; Wang, D.; Zhao, D.; Wang, M. Effects of sulfated polysaccharides from Laminaria japonica on regularating the gut microbiotan and alleviating intestinal inflammation in obese mice. Food Chem. Toxicol. 2022, 168, 113401. [Google Scholar] [CrossRef]
- Murali, A.; Giri, V.; Cameron, H.J.; Sperber, S.; Zickgraf, F.M.; Haake, V.; Driemert, P.; Walk, T.; Kamp, H.; Rietjens, I.M.; et al. Investigating the gut microbiome and metabolome following treatment with artificial sweeteners acesulfame potassium and saccharin in young adult Wistar rats. Food Chem. Toxicol. 2022, 165, 113123. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Genet. 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Gerss, J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011, 30, 215–220. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.-Y.; Li, Y.; Li, J.; Xu, J.; Wu, S.-M.; Chen, T.-X. Dynamic change, influencing factors, and clinical impact of cellular components in human breast milk. Pediatr. Res. 2022, 93, 1765–1771. [Google Scholar] [CrossRef]
- Sangild, P.T.; Vonderohe, C.; Hebib, V.M.; Burrin, D.G. Potential Benefits of Bovine Colostrum in Pediatric Nutrition and Health. Nutrients 2021, 13, 2551. [Google Scholar] [CrossRef] [PubMed]
- Storrø, O.; Øien, T.; Dotterud, C.K.; Jenssen, J.A.; Johnsen, R. A primary health-care intervention on pre- and postnatal risk factor behavior to prevent childhood allergy. The Prevention of Allergy among Children in Trondheim (PACT) study. BMC Public Health 2010, 10, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeyeye, T.E.; Yeung, E.H.; McLain, A.C.; Lin, S.; Lawrence, D.A.; Bell, E.M. Wheeze and food allergies in children born via cesarean delivery: The upstate KIDS study. Am. J. Epidemiol. 2019, 188, 355–362. [Google Scholar] [CrossRef]
- Han, D.H.; Shin, J.-M.; An, S.; Kim, J.S.; Kim, D.-Y.; Moon, S.; Kim, J.-S.; Cho, J.S.; Kim, S.W.; Kim, Y.H.; et al. Long-term Breastfeeding in the Prevention of Allergic Rhinitis: Allergic Rhinitis Cohort Study for Kids (ARCO-Kids Study). Clin. Exp. Otorhinolaryngol. 2019, 12, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kull, I.; Böhme, M.; Wahlgren, C.-F.; Nordvall, L.; Pershagen, G.; Wickman, M. Breast-feeding reduces the risk for childhood eczema. J. Allergy Clin. Immunol. 2005, 116, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, C.D.; Levin, L.; Lemasters, G.; Ryan, P.; Reponen, T.; Villareal, M.; Burkle, J.; Lockey, J.E.; Hershey, G.K.K.; Bernstein, D. Breastfeeding, Aeroallergen Sensitization And Environmental Exposures During Infancy Are Determinants Of Childhood Allergic Rhinitis. J. Allergy Clin. Immunol. 2010, 125, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; García-Esteban, R.; Guxens, M.; Guerra, S.; Mendez, M.; Molto-Puigmarti, C.; Lopez-Sabater, M.C.; Sunyer, J. Effects of prolonged breastfeeding and colostrum fatty acids on allergic manifestations and infections in infancy. Clin. Exp. Allergy 2012, 42, 918–928. [Google Scholar] [CrossRef]
- Antoniou, E.; Dagla, M.; Iliadou, M.; Palaska, E.; Orovou, E. Early Introduction of Solid Foods in Infant’s Nutrition and Long-Term Effects on Childhood: A Systematic Review. Int. J. Innov. Res. Med Sci. 2022, 7, 777–785. [Google Scholar] [CrossRef]
- D’Auria, E.; Borsani, B.; Pendezza, E.; Bosetti, A.; Paradiso, L.; Zuccotti, G.V.; Verduci, E. Complementary feeding: Pitfalls for health outcomes. Int. J. Environ. Res. Public Health 2020, 17, 7931. [Google Scholar] [CrossRef] [PubMed]
- Obbagy, J.E.; English, L.K.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fleischer, D.M.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and food allergy, atopic dermatitis/eczema, asthma, and allergic rhinitis: A systematic review. Am. J. Clin. Nutr. 2019, 109, 890S–934S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, J.A.; Barriga, K.; Leung, D.Y.; Hoffman, M.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics 2006, 117, 2175–2182. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, G.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo Jr, C.A.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Sicherer, S.H.; Wood, R.A.; Stablein, D.; Lindblad, R.; Burks, A.W.; Liu, A.H.; Jones, S.M.; Fleischer, D.M.; Leung, D.Y.; Sampson, H.A. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J. Allergy Clin. Immunol. 2010, 126, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Pitt, T.J.; Becker, A.B.; Chan-Yeung, M.; Chan, E.S.; Watson, W.T.; Chooniedass, R.; Azad, M.B. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. J. Allergy Clin. Immunol. 2018, 141, 620–625.e1. [Google Scholar] [CrossRef] [Green Version]
- Maslova, E.; Granström, C.; Hansen, S.; Petersen, S.B.; Strøm, M.; Willett, W.C.; Olsen, S.F. Peanut and tree nut consumption during pregnancy and allergic disease in children—Should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2012, 130, 724–732. [Google Scholar] [CrossRef]
- Dinakar, C. Anaphylaxis in Children: Current Understanding and Key Issues in Diagnosis and Treatment. Curr. Allergy Asthma Rep. 2012, 12, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Allergic Condition | Effect of Breastfeeding |
|---|---|---|
| Amazouz et al., 2021 [19] | Asthma | Mediterranean diet of mother controls asthma |
| Klop et al., 2017 [20] | Asthma | Protective action against asthma for the first 3 years |
| Ehlayel et al., 2008 [21] | Asthma | protective against respiratory diseases, unlike formula feeding |
| Chui et al. [22] | Eczema | reducing eczema up to the age of 2 years |
| Kull et al., 2005 [40] | Eczema and allergic rhinitis | Breastfeeding for more than 4 months had a lower risk of eczema and allergic rhinitis at 4 years of age |
| Codispoti et al., 2010 [41] | Allergic rhinitis | Prolonged breastfeeding in African American patients reduced the risk of allergic rhinitis at the age of 3 years |
| Morales et al., 2012 [42] | Wheezing, lower respiratory infections and eczema | Exclusive breastfeeding for 4–6 months reduced wheezing, lower respiratory infections and eczema at 7–14 months |
| Storrø edt al., 2010 [37] | Wheezing, asthma and eczema | Longer duration of breastfeeding for at least 6 months was effective in reducing the incidence of eczema, asthma and wheezing at the ages of 1, 2 and 6 years |
| Adeyeye et al., 2019 [38] | Wheezing | Protective against wheezing |
| Han et al., 2019 [39] | Allergic rhinitis | Long term breast feeding (≥12 months) was associated with a reduced risk of allergic rhinitis |
| Järvinen et al., 2019 [4] | Allergic diarrhoea | Breastfeeding by allergen-sensitised mothers may help prevent allergic diarrhoea |
| Study | Solid Food | Guidelines/Recommendations for Introduction |
|---|---|---|
| European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [14] | Solid foods in general | 4–6 months concurrently with breastfeeding |
| European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [14] | Cows’s milk | Strong recommendation to start cow’s milk after the first year |
| European Academy of Allergy and Clinical Immunology (EAACI) [2] | Eggs, cow’s milk and peanuts | Eggs not raw, small quantities (½ egg) 2 times a week from the 4th month Not to supplement with cow’s milk, in order to prevent food allergy in newborns who are breastfed during the first week of life |
| European Academy of Allergy and Clinical Immunology (EAACI) [2] | Peanuts | Peanuts, initially in a safe form with slow administration so as not to block the airways. In local cusines with traditional use of peanuts, introduction from 4 to 11 months while following local guidelines |
| Paediatric Allergy Association [23] | Peanuts | Infants who are at high risk of developing a peanut allergy should be exposed to peanuts at an early age, following an appropriate expert assessment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukou, Z.; Papadopoulou, E.; Panteris, E.; Papadopoulou, S.; Skordou, A.; Karamaliki, M.; Diamanti, E. The Effect of Breastfeeding on Food Allergies in Newborns and Infants. Children 2023, 10, 1046. https://doi.org/10.3390/children10061046
Koukou Z, Papadopoulou E, Panteris E, Papadopoulou S, Skordou A, Karamaliki M, Diamanti E. The Effect of Breastfeeding on Food Allergies in Newborns and Infants. Children. 2023; 10(6):1046. https://doi.org/10.3390/children10061046
Chicago/Turabian StyleKoukou, Zoi, Eleftheria Papadopoulou, Eleftherios Panteris, Styliani Papadopoulou, Anna Skordou, Maria Karamaliki, and Elisavet Diamanti. 2023. "The Effect of Breastfeeding on Food Allergies in Newborns and Infants" Children 10, no. 6: 1046. https://doi.org/10.3390/children10061046