Birth Defects Associated with Prenatal Alcohol Exposure—A Review
Abstract
:1. Introduction
2. Methods
Search Strategy
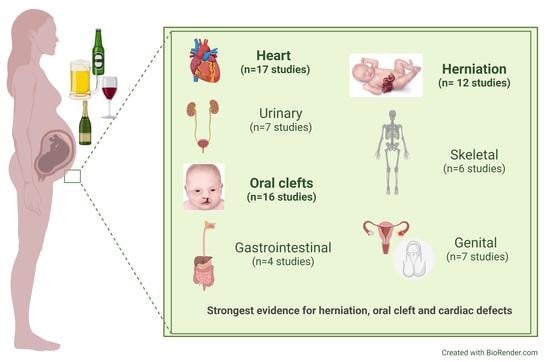
3. Results
3.1. Cardiac Defects
3.1.1. Any Cardiac Defects
3.1.2. Ventricular Septal Defect
3.1.3. Atrial Septal Defect
3.1.4. Conotruncal Heart Defects
3.1.5. Other Heart Defects
3.2. Urinary System Defects
3.3. Oral Clefts
3.3.1. All Oral Clefts
3.3.2. Cleft Lip with or without Cleft Palate or Cleft Palate Only
3.4. Anomalies of Gastrointestinal Tract
3.4.1. Esophageal Atresia
3.4.2. Gastrointestinal Obstruction
3.4.3. Intestinal Atresia/Anal Atresia
3.5. Herniation Defects
3.5.1. Diaphragmatic Hernia
3.5.2. Gastroschisis
3.5.3. Omphalocele
3.6. Neural Tube Defects and Skeletal Defects
Clubfoot, Spina Bifida and Neural Tube Defects
3.7. Anomalies of the Genitals
3.7.1. Cryptorchidism
3.7.2. Hypospadias
3.7.3. Genital Defects
3.8. Other Anomalies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARBD | Alcohol-related birth defect |
| ARND | Alcohol-related neurodevelopmental disorders |
| FASD | Fetal alcohol spectrum disorders |
| FAS | Fetal alcohol syndrome |
| PAE | Prenatal alcohol exposure |
| PFAS | Partial fetal alcohol syndrome |
References
- Reefhuis, J.; Gilboa, S.M.; Anderka, M.; Browne, M.L.; Feldkamp, M.L.; Hobbs, C.A.; Jenkins, M.M.; Langlois, P.H.; Newsome, K.B.; Olshan, A.F.; et al. The National Birth Defects Prevention Study: A Review of the Methods. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 656–669. [Google Scholar] [CrossRef]
- Baldacci, S.; Gorini, F.; Santoro, M.; Pierini, A.; Minichilli, F.; Bianchi, F. Environmental and Individual Exposure and the Risk of Congenital Anomalies: A Review of Recent Epidemiological Evidence TT—Esposizione Ambientale e Individuale e Rischio Di Anomalie Congenite: Una Rassegna Delle Evidenze Epidemiologiche Recenti. Epidemiol. Prev. 2018, 42, 1–34. [Google Scholar] [PubMed]
- World Health Organization. Resolutions and Decisions, Annexes. In Proceedings of the Sixty-Third World Health Assembly, Geneva, Switzerland, 17–21 May 2010. [Google Scholar]
- Harris, B.S.; Bishop, K.C.; Kemeny, H.R.; Walker, J.S.; Rhee, E.; Kuller, J.A. Risk Factors for Birth Defects. Obstet. Gynecol. Surv. 2017, 72, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Smith, D. Recognition of the fetal alcohol syndrome in early infancy. Lancet 1973, 302, 999–1001. [Google Scholar] [CrossRef]
- Lemoine, P.; Harouseau, H.; Borteryu, J.; Menuet, J. Les Enfants Des Parents Alcooliques. Anomalies Observées à Propos de 127 Cas. Quest. Med. 1968, 21, 476–482. [Google Scholar]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.A.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.P.; McGee, C.L. Fetal Alcohol Spectrum Disorders: An Overview with Emphasis on Changes in Brain and Behavior. Exp. Biol. Med. 2005, 230, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.P.; Infante, M.A.; Warren, K.R. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychol. Rev. 2011, 21, 73–80. [Google Scholar] [CrossRef]
- Mattson, S.N.; Bernes, G.A.; Doyle, L.R. Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1046–1062. [Google Scholar] [CrossRef]
- Sivolap, Y.P. Maternal Alcoholism and Its Impact on Child Health. Zhurnal Nevrol. Psihiatr. Im. SS Korsakova 2015, 2015, 133–136. [Google Scholar] [CrossRef]
- Warren, K.R.; Hewitt, B.G.; Thomas, J.D. Fetal Alcohol Spectrum Disorders. Alcohol Res. Health 2011, 34, 4–14. [Google Scholar] [PubMed]
- Pruett, D.; Waterman, E.H.; Caughey, A.B. Fetal Alcohol Exposure: Consequences, Diagnosis, and Treatment. Obstet. Gynecol. Surv. 2013, 68, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Denny, L.A.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.G. Diagnostic Criteria for Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Arch. Argent. Pediatría 2010, 108, 61–67. [Google Scholar]
- Williams, J.F.; Smith, V.C. Fetal Alcohol Spectrum Disorders. Pediatrics 2015, 136, 1395–1406. [Google Scholar] [CrossRef]
- Cook, J.L.; Green, C.R.; Lilley, C.M.; Anderson, S.M.; Baldwin, M.E.; Chudley, A.E.; Conry, J.L.; LeBlanc, N.; Loock, C.A.; Lutke, J.; et al. Fetal Alcohol Spectrum Disorder: A Guideline for Diagnosis across the Lifespan. CMAJ 2016, 188, 191–197. [Google Scholar] [CrossRef]
- Astley, S.J. Palpebral Fissure Length Measurement: Accuracy of the FAS Facial Photographic Analysis Software and Inaccuracy of the Ruler. J. Popul. Ther. Clin. Pharmacol. 2015, 22, e9–e26. [Google Scholar]
- Blackston, R.D.; Coles, C.D.; Kable, J.A. Evidence for Severity of Dysmorphology in Fetal Alcohol Syndrome and Direct Correlation with Developmental, Behavioral, Social and Educational Outcomes and to Psychotropic Medications; University of Iowa: Iowa City, IA, USA, 2005. [Google Scholar]
- Goodlett, C.R.; Horn, K.H. Mechanisms of Alcohol-Induced Damage to the Developing Nervous System. Alcohol Res. Health 2001, 25, 175–184. [Google Scholar]
- Trikalinos, T. Abstrackr: Software for Semi-Automatic Citation Screening; Effective Health Care Program, Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [Google Scholar]
- Tikkanen, J.; Heinonen, O.P. Maternal Exposure to Chemical and Physical Factors during Pregnancy and Cardiovascular Malformations in the Offspring. Teratology 1991, 43, 591–600. [Google Scholar] [CrossRef]
- McDonald, A.D.; Armstrong, B.G.; Sloan, M. Cigarette, Alcohol, and Coffee Consumption and Congenital Defects. Am. J. Public Health 1992, 82, 91–93. [Google Scholar] [CrossRef]
- Cedergren, M.I.; Selbing, A.J.; Kallen, B.A.J. Risk Factors for Cardiovascular Malformation—A Study Based on Prospectively Collected Data. Scand. J. Work. Environ. Health 2002, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Frías, M.L.; Bermejo, E.; Rodríguez-Pinilla, E.; Frías, J.L. Risk for Congenital Anomalies Associated with Different Sporadic and Daily Doses of Alcohol Consumption during Pregnancy: A Case-Control Study. Birth Defects Res. Part A Clin. Mol. Teratol. 2004, 70, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Cleves, M.A.; Honein, M.A.; Romitti, P.A.; Botto, L.D.; Yang, S.; Hobbs, C.A. Maternal Smoking and Congenital Heart Defects. Pediatrics 2008, 121, e810–e816. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; MacLeod, S.L.; Jill James, S.; Cleves, M.A. Congenital Heart Defects and Maternal Genetic, Metabolic, and Lifestyle Factors. Birth Defects Res. A. Clin. Mol. Teratol. 2011, 91, 195–203. [Google Scholar] [CrossRef]
- Mateja, W.A.; Nelson, D.B.; Kroelinger, C.D.; Ruzek, S.; Segal, J. The Association between Maternal Alcohol Use and Smoking in Early Pregnancy and Congenital Cardiac Defects. J. Women’s Health 2012, 21, 26–34. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, J.; Liang, C.; Zhao, Y.; Chen, F.; Wang, D.; Pei, L. Geographical Variations in Maternal Lifestyles during Pregnancy Associated with Congenital Heart Defects among Live Births in Shaanxi Province, Northwestern China. Sci. Rep. 2020, 10, 12958. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Kang, Y.; Cheng, Y.; Yan, H. The Association of Maternal Lifestyle with Birth Defects in Shaanxi Province, Northwest China. PLoS ONE 2015, 10, e0139452. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Joseph, K.S.; Lisonkova, S.; Rouleau, J.; Van den Hof, M.; Sauve, R.; Kramer, M.S. Association between Maternal Chronic Conditions and Congenital Heart Defects: A Population-Based Cohort Study. Circulation 2013, 128, 583–589. [Google Scholar] [CrossRef]
- Kurita, H.; Motoki, N.; Inaba, Y.; Misawa, Y.; Ohira, S.; Kanai, M.; Tsukahara, T.; Nomiyama, T.; Kamijima, M.; Yamazaki, S.; et al. Maternal Alcohol Consumption and Risk of Offspring with Congenital Malformation: The Japan Environment and Children’s Study. Pediatr. Res. 2021, 90, 479. [Google Scholar] [CrossRef]
- Williams, L.J.; Correa, A.; Rasmussen, S. Maternal Lifestyle Factors and Risk for Ventricular Septal Defects. Birth Defects Res. Part Clin. Mol. Teratol. 2004, 70, 59–64. [Google Scholar] [CrossRef]
- Strandberg-Larsen, K.; Skov-Ettrup, L.S.; Gronbaek, M.; Andersen, A.-M.N.; Olsen, J.; Tolstrup, J. Maternal Alcohol Drinking Pattern during Pregnancy and the Risk for an Offspring with an Isolated Congenital Heart Defect and in Particular a Ventricular Septal Defect or an Atrial Septal Defect. Birth Defects Res. Part Clin. Mol. Teratol. 2011, 91, 616–622. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.M.; Elliott, E.J.; Nassar, N.; Bower, C. Exploring the Potential to Use Data Linkage for Investigating the Relationship between Birth Defects and Prenatal Alcohol Exposure. Birth Defects Res. Part Clin. Mol. Teratol. 2013, 97, 497–504. [Google Scholar] [CrossRef]
- Kovalenko, A.; Anda, E.; Odland, J.; Nieboer, E.; Brenn, T.; Krettek, A. Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study. Int. J. Environ. Res. Public Health 2018, 15, 1320. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Shaw, G.M.; Yang, W.; Lammer, E.J. Maternal Periconceptional Alcohol Consumption and Risk for Conotruncal Heart Defects. Birth Defects Res. Part Clin. Mol. Teratol. 2003, 67, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.C.; Baer, R.J.; Bandoli, G.; Chambers, C.D.; Jelliffe-Pawlowski, L.L.; Ram Kumar, S. Association of Alcohol Use Diagnostic Codes in Pregnancy and Offspring Conotruncal and Endocardial Cushion Heart Defects. J. Am. Heart Assoc. 2022, 11, e022175. [Google Scholar] [CrossRef]
- Steinberger, E.K.; Ferencz, C.; Loffredo, C.A. Infants with Single Ventricle: A Population-Based Epidemiological Study. Teratology 2002, 65, 106–115. [Google Scholar] [CrossRef]
- Moore, C.A.; Khoury, M.J.; Liu, Y. Does Light-to-Moderate Alcohol Consumption during Pregnancy Increase the Risk for Renal Anomalies among Offspring? Pediatrics 1997, 99, e11. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; McCall, D.; Engelman, C.; Schrier, R.W. Congenital Renal Agenesis: Case-Control Analysis of Birth Characteristics. Am. J. Kidney Dis. 2002, 39, 689–694. [Google Scholar] [CrossRef]
- Slickers, J.E.; Olshan, A.F.; Siega-Riz, A.M.; Honein, M.A.; Aylsworth, A.S. Maternal Body Mass Index and Lifestyle Exposures and the Risk of Bilateral Renal Agenesis or Hypoplasia: The National Birth Defects Prevention Study. Am. J. Epidemiol. 2008, 168, 1259–1267. [Google Scholar] [CrossRef]
- Groen In’t Woud, S.; Renkema, K.Y.; Schreuder, M.F.; Wijers, C.H.W.; van der Zanden, L.F.M.; Knoers, N.V.A.M.; Feitz, W.F.J.; Bongers, E.M.H.F.; Roeleveld, N.; van Rooij, I.A.L.M. Maternal Risk Factors Involved in Specific Congenital Anomalies of the Kidney and Urinary Tract: A Case-Control Study. Birth Defects Res. Part Clin. Mol. Teratol. 2016, 106, 596–603. [Google Scholar] [CrossRef]
- Merritt, L. Part 1. Understanding the Embryology and Genetics of Cleft Lip and Palate. Adv. Neonatal Care 2005, 5, 64–71. [Google Scholar] [CrossRef]
- Werler, M.M.; Lammer, E.J.; Rosenberg, L.; Mitchell, A.A. Maternal Alcohol Use in Relation to Selected Birth Defects. Am. J. Epidemiol. 1991, 134, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Munger, R.G.; Romitti, P.A.; Daack-Hirsch, S.; Burns, T.L.; Murray, J.C.; Hanson, J. Maternal Alcohol Use and Risk of Orofacial Cleft Birth Defects. Teratology 1996, 54, 27–33. [Google Scholar] [CrossRef]
- Shaw, G.M.; Lammer, E.J. Maternal periconceptional alcohol consumption and risk for orofacial clefts. J. Pediatr. 1999, 134, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lorente; Cordier; Goujard; Ayme; Bianchi; Calzolari; DeWalle; Knill–Jones Tobacco and Alcohol Use during Pregnancy and Risk of Oral Clefts. Am. J. Public Heal. 2000, 90, 415–419.
- Beaty, T.H.; Wang, H.; Hetmanski, J.B.; Fan, Y.T.; Zeiger, J.S.; Liang, K.Y.; Chiu, Y.F.; Vanderkolk, C.A.; Seifert, K.C.; Wulfsberg, E.A.; et al. A Case-Control Study of Nonsyndromic Oral Clefts in Maryland. Ann. Epidemiol. 2001, 11, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Werler, M.M.; Hayes, C.; Mitchell, A.A. Low Maternal Alcohol Consumption during Pregnancy and Oral Clefts in Offspring: The Slone Birth Defects Study. Birth Defects Res. Part A Clin. Mol. Teratol. 2003, 67, 509–514. [Google Scholar] [CrossRef]
- Chevrier, C.; Perret, C.; Bahuau, M.; Nelva, A.; Herman, C.; Francannet, C.; Robert-Gnansia, E.; Cordier, S. Interaction between the ADH1C Polymorphism and Maternal Alcohol Intake in the Risk of Nonsyndromic Oral Clefts: An Evaluation of the Contribution of Child and Maternal Genotypes. Birth Defects Res. Part Clin. Mol. Teratol. 2005, 73, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Romitti, P.A.; Sun, L.; Honein, M.A.; Reefhuis, J.; Correa, A.; Rasmussen, S.A. Maternal Periconceptional Alcohol Consumption and Risk of Orofacial Clefts. Am. J. Epidemiol. 2007, 166, 775–785. [Google Scholar] [CrossRef]
- Bille, C.; Olsen, J.; Vach, W.; Knudsen, V.K.; Olsen, S.F.; Rasmussen, K.; Murray, J.C.; Andersen, A.M.N.; Christensen, K. Oral Clefts and Life Style Factors—A Case-Cohort Study Based on Prospective Danish Data. Eur. J. Epidemiol. 2007, 22, 173–181. [Google Scholar] [CrossRef]
- DeRoo, L.A.; Wilcox, A.J.; Drevon, C.A.; Lie, R.T. First-Trimester Maternal Alcohol Consumption and the Risk of Infant Oral Clefts in Norway: A Population-Based Case-Control Study. Am. J. Epidemiol. 2008, 168, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.C.G.; Koifman, S. Oral Clefts, Consanguinity, Parental Tobacco and Alcohol Use: A Case-Control Study in Rio de Janeiro, Brazil. Braz. Oral Res. 2009, 23, 31–37. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshioka, E.; Saijo, Y.; Miyamoto, T.; Sengoku, K.; Azuma, H.; Tanahashi, Y.; Ito, Y.; Kobayashi, S.; Minatoya, M.; et al. Population Attributable Fractions of Modifiable Risk Factors for Nonsyndromic Orofacial Clefts: A Prospective Cohort Study From the Japan Environment and Children’s Study. J. Epidemiol. 2021, 31, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, R.; Li, X.; Mo, X. Environmental Factors in the Etiology of Isolated and Nonisolated Esophageal Atresia in a Chinese Population: A Case-Control Study. Birth Defects Res. Part Clin. Mol. Teratol. 2016, 106, 840–846. [Google Scholar] [CrossRef]
- Wong-Gibbons, D.L.; Romitti, P.A.; Sun, L.; Moore, C.A.; Reefhuis, J.; Bell, E.M.; Olshan, A.F. Maternal Periconceptional Exposure to Cigarette Smoking and Alcohol and Esophageal Atresia +/- Tracheo-Esophageal Fistula. Birth Defects Res. Part Clin. Mol. Teratol. 2008, 82, 776–784. [Google Scholar] [CrossRef]
- Mangyanda, M.K.; Mbuila, C.; Geniez, L.; Personne, A.; Boize, P.; Gasmi, E.H.; Saf, E.; Hayat, P. Fetal alcohol syndrome and hypertrophic pyloric stenosis in two brothers. Arch. Pediatr. 1998, 5, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.K.; Satodia, P.; Whyte, H. Fetal Alcohol Syndrome and Pyloric Stenosis: Alcohol Induced or an Association? J. Perinat. Med. 2005, 33, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, P. Fetal alcohol syndrome and pyloric stenosis. Arch. Pediatr. 1999, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Caspers, K.M.; Oltean, C.; Romitti, P.A.; Sun, L.; Pober, B.R.; Rasmussen, S.A.; Yang, W.; Druschel, C. Maternal Periconceptional Exposure to Cigarette Smoking and Alcohol Consumption and Congenital Diaphragmatic Hernia. Birth Defects Res. Part Clin. Mol. Teratol. 2010, 88, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.; Suhl, J.; Kancherla, V.; Conway, K.M.; Oleson, J.; Sidhu, A.; Nestoridi, E.; Fisher, S.C.; Rasmussen, S.A.; Yang, W.; et al. Maternal Cigarette Smoking and Alcohol Consumption and Congenital Diaphragmatic Hernia. Birth Defects Res. 2022, 114, 746–758. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Shapiro, S. Demographic, Reproductive, Medical, and Environmental Factors in Relation to Gastroschisis. Teratology 1992, 45, 353–360. [Google Scholar] [CrossRef]
- Salomon, J.A.; Vos, T.; Hogan, D.R.; Gagnon, M.; Naghavi, M.; Mokdad, A.; Begum, N.; Shah, R.; Karyana, M.; Kosen, S.; et al. Common Values in Assessing Health Outcomes from Disease and Injury: Disability Weights Measurement Study for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2129–2143. [Google Scholar] [CrossRef] [PubMed]
- Paranjothy, S.; Broughton, H.; Evans, A.; Huddart, S.; Drayton, M.; Jefferson, R.; Rankin, J.; Draper, E.; Cameron, A.; Palmer, S.R. The Role of Maternal Nutrition in the Aetiology of Gastroschisis: An Incident Case-Control Study. Int. J. Epidemiol. 2012, 41, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.R.; Evans, A.; Broughton, H.; Huddart, S.; Drayton, M.; Rankin, J.; Draper, E.S.; Cameron, A.; Paranjothy, S. The Role of Maternal Stress in Early Pregnancy in the Aetiology of Gastroschisis: An Incident Case Control Study. PLoS ONE 2013, 8, e80103. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Aceves, M.; Bobadilla-Morales, L.; Mellin-Sanchez, E.L.; Corona-Rivera, A.; Perez-Molina, J.J.; Cardenas-Ruiz Velasco, J.J.; Corona-Rivera, J.R. Prevalence and Risk Factors for Gastroschisis in a Public Hospital from West Mexico. Congenit. Anom. 2015, 55, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Werler, M.M.; Guéry, E.; Waller, D.K.; Parker, S.E. Gastroschisis and Cumulative Stressor Exposures. Epidemiology 2018, 29, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, L.C.; Kalia, N.; Vazquez, J.; Hilton, S.A.; Zaretsky, M.V.; Behrendt, N.; Galan, H.L.; Marwan, A.I.; Liechty, K.W. Determining Maternal Risk Factors for Gastroschisis Using Colorado’s Birth Registry Database. J. Pediatr. Surg. 2020, 55, 1002–1005. [Google Scholar] [CrossRef]
- Mac Bird, T.; Robbins, J.M.; Druschel, C.; Cleves, M.A.; Yang, S.; Hobbs, C.A. Demographic and Environmental Risk Factors for Gastroschisis and Omphalocele in the National Birth Defects Prevention Study. J. Pediatr. Surg. 2009, 44, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- McAteer, J.P.; Hecht, A.; De Roos, A.J.; Goldin, A.B. Maternal Medical and Behavioral Risk Factors for Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2014, 49, 34–38; discussion 38. [Google Scholar] [CrossRef] [PubMed]
- Rittler, M.; Campana, H.; Ermini, M.L.; Gili, J.A.; Poletta, F.A.; Pawluk, M.S.; Gimenez, L.G.; Cosentino, V.R.; Castilla, E.E.; Lopez-Camelo, J.S. Gastroschisis and Young Mothers: What Makes Them Different from Other Mothers of the Same Age? Birth Defects Res. Part Clin. Mol. Teratol. 2015, 103, 536–543. [Google Scholar] [CrossRef]
- Richardson, S.; Browne, M.L.; Rasmussen, S.A.; Druschel, C.M.; Sun, L.; Jabs, E.W.; Romitti, P.A. Associations between Periconceptional Alcohol Consumption and Craniosynostosis, Omphalocele, and Gastroschisis. Birth Defects Res. Part Clin. Mol. Teratol. 2011, 91, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Werler, M.M.; Yazdy, M.M.; Kasser, J.R.; Mahan, S.T.; Meyer, R.E.; Anderka, M.; Druschel, C.M.; Mitchell, A.A. Maternal Cigarette, Alcohol, and Coffee Consumption in Relation to Risk of Clubfoot. Paediatr. Perinat. Epidemiol. 2015, 29, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Benedum, C.M.; Yazdy, M.M.; Mitchell, A.A.; Werler, M.M. Risk of Spina Bifida and Maternal Cigarette, Alcohol, and Coffee Use during the First Month of Pregnancy. Int. J. Environ. Res. Public Health 2013, 10, 3263–3281. [Google Scholar] [CrossRef] [PubMed]
- Makelarski, J.A.; Romitti, P.A.; Sun, L.; Burns, T.L.; Druschel, C.M.; Suarez, L.; Olshan, A.F.; Siega-Riz, A.M.; Olney, R.S. Periconceptional Maternal Alcohol Consumption and Neural Tube Defects. Birth Defects Res. Part Clin. Mol. Teratol. 2013, 97, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.; Felkner, M.; Brender, J.D.; Canfield, M.; Hendricks, K. Maternal Exposures to Cigarette Smoke, Alcohol, and Street Drugs and Neural Tube Defect Occurrence in Offspring. Matern. Child Health J. 2008, 12, 394–401. [Google Scholar] [CrossRef]
- Louden, A.R.; Suhl, J.; Kancherla, V.; Caspers Conway, K.M.; Makelarski, J.; Howley, M.M.; Hoyt, A.T.; Olney, R.S.; Olshan, A.F.; Romitti, P.A. Association between Maternal Periconceptional Alcohol Consumption and Neural Tube Defects: Findings from the National Birth Defects Prevention Study, 1997–2011. Birth Defects Res. 2020, 112, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Carbone, P.; Giordano, F.; Nori, F.; Mantovani, A.; Taruscio, D.; Lauria, L.; Figa-Talamanca, I. The Possible Role of Endocrine Disrupting Chemicals in the Aetiology of Cryptorchidism and Hypospadias: A Population-Based Case-Control Study in Rural Sicily. Int. J. Androl. 2007, 30, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Strandberg-Larsen, K.; Jensen, M.S.; Ramlau-Hansen, C.H.; Gronbaek, M.; Olsen, J. Alcohol Binge Drinking during Pregnancy and Cryptorchidism. Hum. Reprod. 2009, 24, 3211–3219. [Google Scholar] [CrossRef]
- Jensen, M.S.; Bonde, J.P.; Olsen, J. Prenatal Alcohol Exposure and Cryptorchidism. Acta Paediatr. 2007, 96, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, I.N.; Jensen, T.K.; Petersen, J.H.; Skakkebaek, N.E.; Toppari, J.; Main, K.M. Cryptorchidism and Maternal Alcohol Consumption during Pregnancy. Environ. Health Perspect. 2007, 115, 272–277. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing Guideline Development, Reporting and Evaluation in Health Care. CMAJ 2010, 182, E839-42. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, V.; Romitti, P.A.; Sun, L.; Carey, J.C.; Burns, T.L.; Siega-Riz, A.M.; Druschel, C.M.; Lin, A.E.; Olney, R.S. Descriptive and Risk Factor Analysis for Choanal Atresia: The National Birth Defects Prevention Study, 1997–2007. Eur. J. Med. Genet. 2014, 57, 220–229. [Google Scholar] [CrossRef] [PubMed]
- McQuire, C.; Daniel, R.; Hurt, L.; Kemp, A.; Paranjothy, S. The Causal Web of Foetal Alcohol Spectrum Disorders: A Review and Causal Diagram. Eur. Child Adolesc. Psychiatry 2020, 29, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Olsen, J.; Norgaard-Pedersen, B.; Basso, O.; Stovring, H.; Milhollin-Johnson, L.; Murray, J.C. Oral Clefts, Transforming Growth Factor Alpha Gene Variants, and Maternal Smoking: A Population-Based Case-Control Study in Denmark, 1991–1994. Am. J. Epidemiol. 1999, 149, 248–255. [Google Scholar] [CrossRef] [PubMed]
| Birth Defect | Type of Study | Number of Studies a | Significant Findings from All Studies (n, (%)) | Timing of Alcohol Exposure Assessment (n, (%)) | Significant Findings Among Papers with Periconceptional Exposure (n, (%)) | ||
|---|---|---|---|---|---|---|---|
| Case Control | Cohort | Cross Sectional | p = 0.05 | Periconceptional-First Trimester | p = 0.05 | ||
| Heart | 11 | 4 | 2 | 17 | 8 (47%) | 9 (53%) | 2 (22%) |
| Urinary | 6 | 1 | 0 | 7 | 2 (28%) | 4 (57%) | 1 (25%) |
| Oral clefts | 13 | 2 | 1 | 16 | 8 (50%) | 12 (75%) | 7 (58%) |
| Gastrointestinal | 3 | 1 | 0 | 4 | 1 (25%) | 2 (50%) | 1 (50%) |
| Hernia | 12 | 0 | 0 | 12 | 8 (58%) | 10 (83%) | 6 (60%) |
| Skeletal | 6 | 0 | 0 | 6 | 0 (0%) | 6 (100%) | 0 (0%) |
| Genital | 5 | 2 | 0 | 7 | 2 (29%) | 2 (29%) | 2 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyląg, K.A.; Anunziata, F.; Bandoli, G.; Chambers, C. Birth Defects Associated with Prenatal Alcohol Exposure—A Review. Children 2023, 10, 811. https://doi.org/10.3390/children10050811
Dyląg KA, Anunziata F, Bandoli G, Chambers C. Birth Defects Associated with Prenatal Alcohol Exposure—A Review. Children. 2023; 10(5):811. https://doi.org/10.3390/children10050811
Chicago/Turabian StyleDyląg, Katarzyna Anna, Florencia Anunziata, Gretchen Bandoli, and Christina Chambers. 2023. "Birth Defects Associated with Prenatal Alcohol Exposure—A Review" Children 10, no. 5: 811. https://doi.org/10.3390/children10050811