The Evolution of Safe and Effective Coaguligands for Vascular Targeting and Precision Thrombosis of Solid Tumors and Vascular Malformations
Abstract
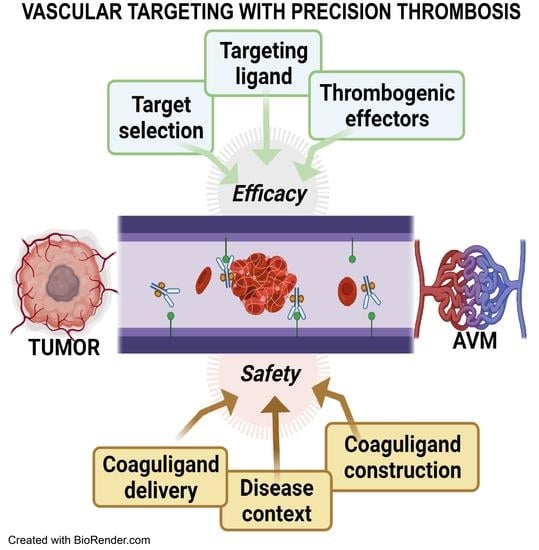
:1. Introduction—The Origins of Vascular Targeting for Precision Thrombosis
2. Part I—Vascular Targets: Balancing Specificity against Efficacy
2.1. MHCII
2.2. VCAM-1
2.3. Phosphatidylserine (PS)
2.4. Vascular Endothelial Growth Factor (VEGF) Receptors
2.5. Integrins and Integrin Receptors
2.6. Matrix Targeting
2.7. Translocated Intracellular Proteins
2.8. Targets in Human Translation
2.9. Summary—Part I
3. Part II—The Importance of Thrombogenic Effectors in Precision Thrombosis
3.1. Tissue Factor (TF)
3.2. Approaches to Increase TF Coaguligand Efficacy
3.3. Thrombin
3.4. Truncated Coagulase (tCoa)
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cruz, E.; Kayser, V. Monoclonal antibody therapy of solid tumors: Clinical limitations and novel strategies to enhance treatment efficacy. Biologics 2019, 13, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, P.E.; Burrows, F.J. Antibody-directed targeting of the vasculature of solid tumors. Breast Cancer Res. Treat. 1995, 36, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Burrows, F.J.; Overholser, J.P.; Thorpe, P.E. Potent antitumor effects of an antitumor endothelial cell immunotoxin in a murine vascular targeting model. Cell Biophys. 1994, 24–25, 15–25. [Google Scholar] [CrossRef]
- Burrows, F.J.; Thorpe, P.E. Vascular targeting—A new approach to the therapy of solid tumors. Pharmacol. Ther. 1994, 64, 155–174. [Google Scholar] [CrossRef]
- Burrows, F.J.; Watanabe, Y.; Thorpe, P.E. A murine model for antibody-directed targeting of vascular endothelial cells in solid tumors. Cancer Res. 1992, 52, 5954–5962. [Google Scholar]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Folkman, J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann. Surg. 1972, 175, 409–416. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupo, G.; Caporarello, N.; Olivieri, M.; Cristaldi, M.; Motta, C.; Bramanti, V.; Avola, R.; Salmeri, M.; Nicoletti, F.; Anfuso, C.D. Anti-angiogenic therapy in cancer: Downsides and new pivots for precision medicine. Front. Pharmacol. 2017, 7, 519. [Google Scholar] [CrossRef] [Green Version]
- Denekamp, J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br. J. Cancer 1982, 45, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Schliemann, C.; Neri, D. Antibody-based vascular tumor targeting. Recent Results Cancer Res. 2010, 180, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.Q.; Lee, P.; Lin, H.; Soker, S.; Klagsbrun, M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000, 14, 2532–2539. [Google Scholar] [CrossRef] [Green Version]
- Dreischalück, J.; Schwöppe, C.; Spieker, T.; Kessler, T.; Tiemann, K.; Liersch, R.; Schliemann, C.; Kreuter, M.; Kolkmeyer, A.; Hintelmann, H. Vascular infarction by subcutaneous application of tissue factor targeted to tumor vessels with NGR-peptides: Activity and toxicity profile. Int. J. Oncol. 2010, 37, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Stucke-Ring, J.; Ronnacker, J.; Brand, C.; Holtke, C.; Schliemann, C.; Kessler, T.; Schmidt, L.H.; Harrach, S.; Mantke, V.; Hintelmann, H.; et al. Combinatorial effects of doxorubicin and retargeted tissue factor by intratumoral entrapment of doxorubicin and proapoptotic increase of tumor vascular infarction. Oncotarget 2016, 7, 82458–82472. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Molema, G.; King, S.; Watkins, L.; Edgington, T.S.; Thorpe, P.E. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997, 275, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, S.; Gao, B.; Duffy, S.; Watkins, L.; Rote, N.; Thorpe, P.E. Infarction of solid Hodgkin’s tumors in mice by antibody-directed targeting of tissue factor to tumor vasculature. Cancer Res. 1998, 58, 4646–4653. [Google Scholar] [PubMed]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef]
- Osbun, J.W.; Reynolds, M.R.; Barrow, D.L. Arteriovenous malformations: Epidemiology, clinical presentation, and diagnostic evaluation. Handb. Clin. Neurol. 2017, 143, 25–29. [Google Scholar] [CrossRef]
- Achrol, A.S.; Guzman, R.; Varga, M.; Adler, J.R.; Steinberg, G.K.; Chang, S.D. Pathogenesis and radiobiology of brain arteriovenous malformations: Implications for risk stratification in natural history and posttreatment course. Neurosurg. Focus 2009, 26, E9. [Google Scholar] [CrossRef]
- Goldberg, J.; Raabe, A.; Bervini, D. Natural history of brain arteriovenous malformations: Systematic review. J. Neurosurg. Sci. 2018, 62, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Heim, A.D.; Boltshauser, E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003, 25, 416–421. [Google Scholar] [CrossRef]
- Moftakhar, P.; Hauptman, J.S.; Malkasian, D.; Martin, N.A. Cerebral arteriovenous malformations. Part 2: Physiology. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef]
- Friedman, W.A.; Bova, F.J. Radiosurgery for arteriovenous malformations. Neurol. Res. 2011, 33, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Dienst, A.; Grunow, A.; Unruh, M.; Rabausch, B.; Nor, J.E.; Fries, J.W.; Gottstein, C. Specific occlusion of murine and human tumor vasculature by VCAM-1-targeted recombinant fusion proteins. J. Natl. Cancer Inst. 2005, 97, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.; Chin, B.S.; Blann, A.D. Cancer and the prothrombotic state. Lancet Oncol. 2002, 3, 27–34. [Google Scholar] [CrossRef]
- Ran, S.; Thorpe, P.E. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1479–1484. [Google Scholar] [CrossRef]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Leventis, P.A.; Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.R.; Gibson, D.F.; Schwartz, S.M.; Tait, J.F. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ. Res. 1995, 77, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ding, W.Q.; Vaught, J.L.; Wolf, R.F.; Morrissey, J.H.; Harrison, R.G.; Lind, S.E. A soluble tissue factor-annexin V chimeric protein has both procoagulant and anticoagulant properties. Blood 2006, 107, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Belzile, O.; Huang, X.; Gong, J.; Carlson, J.; Schroit, A.J.; Brekken, R.A.; Freimark, B.D. Antibody targeting of phosphatidylserine for the detection and immunotherapy of cancer. ImmunoTargets Ther. 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storer, K.; Tu, J.; Karunanayaka, A.; Smee, R.; Short, R.; Thorpe, P.; Stoodley, M. Coadministration of low-dose lipopolysaccharide and soluble tissue factor induces thrombosis after radiosurgery in an animal arteriovenous malformation model. Neurosurgery 2007, 61, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Duong, T.T.; Fairhall, J.M.; Smee, R.I.; Stoodley, M.A. Durable thrombosis in a rat model of arteriovenous malformation treated with radiosurgery and vascular targeting. J. Neurosurg. 2014, 120, 113–119. [Google Scholar] [CrossRef]
- Zhao, Z.; Johnson, M.S.; Chen, B.; Grace, M.; Ukath, J.; Lee, V.S.; McRobb, L.S.; Sedger, L.M.; Stoodley, M.A. Live-cell imaging to detect phosphatidylserine externalization in brain endothelial cells exposed to ionizing radiation: Implications for the treatment of brain arteriovenous malformations. J. Neurosurg. 2016, 124, 1780–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoufi Rad, N.; McRobb, L.S.; Zhao, Z.; Lee, V.S.; Patel, N.J.; Qureshi, A.S.; Grace, M.; McHattan, J.J.; Amal Raj, J.V.; Duong, H.; et al. Phosphatidylserine Translocation after Radiosurgery in an Animal Model of Arteriovenous Malformation. Radiat. Res. 2017, 187, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Ugoya, S.O.; Zhao, Z.; McRobb, L.S.; Grau, G.E.; Combes, V.; Inglis, D.W.; Gauden, A.J.; Lee, V.S.; Moutrie, V.; et al. Stable thrombus formation on irradiated microvascular endothelial cells under pulsatile flow: Pre-testing annexin V-thrombin conjugate for treatment of brain arteriovenous malformations. Thromb. Res. 2018, 167, 104–112. [Google Scholar] [CrossRef]
- He, J.; Luster, T.A.; Thorpe, P.E. Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin. Cancer Res. 2007, 13, 5211–5218. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yin, Y.; Luster, T.A.; Watkins, L.; Thorpe, P.E. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin. Cancer Res. 2009, 15, 6871–6880. [Google Scholar] [CrossRef] [Green Version]
- Storer, K.P.; Tu, J.; Karunanayaka, A.; Morgan, M.K.; Stoodley, M.A. Thrombotic molecule expression in cerebral vascular malformations. J. Clin. Neurosci. 2007, 14, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; Dewit, L.G.; Boomgaard, M.N.; Brinkman, H.J.; van Mourik, J.A. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor. Radiat. Res. 1994, 137, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, Y.; Li, P.; Bai, X.; Ruan, C. Thrombomodulin as a marker of radiation-induced endothelial cell injury. Radiat. Res. 1992, 131, 285–289. [Google Scholar] [CrossRef]
- Gauden, A.J.; McRobb, L.S.; Lee, V.S.; Subramanian, S.; Moutrie, V.; Zhao, Z.; Stoodley, M.A. Occlusion of Animal Model Arteriovenous Malformations Using Vascular Targeting. Transl. Stroke Res. 2020, 11, 689–699. [Google Scholar] [CrossRef]
- Kashba, S.R.; Patel, N.J.; Grace, M.; Lee, V.S.; Raoufi-Rad, N.; Amal Raj, J.V.; Duong, T.T.; Stoodley, M. Angiographic, hemodynamic, and histological changes in an animal model of brain arteriovenous malformations treated with Gamma Knife radiosurgery. J. Neurosurg. 2015, 123, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, V.; Gautier, B.; Garbay, C.; Vidal, M.; Inguimbert, N. Development of a chemiluminescent screening assay for detection of vascular endothelial growth factor receptor 1 ligands. Anal. Biochem. 2007, 366, 108–110. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, M.; Hanby, A.M.; Stamp, G.W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J. Pathol. 1991, 165, 25–32. [Google Scholar] [CrossRef]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 2010, 16, 3548–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Targeted pharmacotherapy of retinal diseases with ranibizumab. Drugs Today 2007, 43, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.; Shima, D.T.; Calias, P.; Cunningham, E.T.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.; Borgstrom, P.; Bhattacharjee, G.; Belting, M.; Edgington, T.S. A selective tumor microvasculature thrombogen that targets a novel receptor complex in the tumor angiogenic microenvironment. Cancer Res. 2005, 65, 11109–11117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.Y.; Li, Y.N.; Wang, H.; Huang, Y.H.; Lin, Y.Y.; Tan, G.H. A fusion protein containing murine vascular endothelial growth factor and tissue factor induces thrombogenesis and suppression of tumor growth in a colon carcinoma model. J. Zhejiang Univ. Sci. B 2008, 9, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Ye, M.; Wang, X.; Li, Z.; Chen, X.; Dou, X.; Dai, Y.; Zeng, F.; Luo, L.; Wang, C.; et al. A recombined fusion protein SP5.2/tTF induce thrombosis in tumor blood vessel. Neoplasma 2015, 62, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zou, M.; Wang, S.; Wang, L.; Wang, L.; Luo, F.; Wu, T.; Yan, J. Preparation of truncated tissue factor antineuropilin-1 monoclonal antibody conjugate and identification of its selective thrombosis in tumor blood vessels. Anticancer Drugs 2019, 30, 441–450. [Google Scholar] [CrossRef]
- Zou, M.; Samiullah, M.; Xu, P.; Wang, S.; He, J.; Wu, T.; Luo, F.; Yan, J. Construction of novel procoagulant protein targeting neuropilin-1 on tumour vasculature for tumour embolization therapy. J. Drug Target. 2019, 27, 885–895. [Google Scholar] [CrossRef]
- Qiu, G.; Xie, X.; Zhao, B.; Xu, L.; Chen, Y. Fusion protein tTF-EG3287 induces occlusion of tumor vessels and impairs tumor growth in human colon cancer. Neoplasma 2019, 66, 252–260. [Google Scholar] [CrossRef]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Erdreich-Epstein, A.; Shimada, H.; Groshen, S.; Liu, M.; Metelitsa, L.S.; Kim, K.S.; Stins, M.F.; Seeger, R.C.; Durden, D.L. Integrins αvβ3 and αvβ5 are expressed by endothelium of high-risk neuroblastoma and their inhibition is associated with increased endogenous ceramide. Cancer Res. 2000, 60, 712–721. [Google Scholar]
- Carson-Walter, E.B.; Watkins, D.N.; Nanda, A.; Vogelstein, B.; Kinzler, K.W.; St Croix, B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001, 61, 6649–6655. [Google Scholar] [PubMed]
- Fernando, S.; Fletcher, B.S. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer Res. 2009, 69, 5126–5132. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Gay, D.A.; Ruoslahti, E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J. Biol. Chem. 1993, 268, 20205–20210. [Google Scholar] [CrossRef]
- Koivunen, E.; Wang, B.; Ruoslahti, E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J. Cell Biol. 1994, 124, 373–380. [Google Scholar] [CrossRef]
- Ruoslahti, E. Fibronectin and its receptors. Annu. Rev. Biochem. 1988, 57, 375–413. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashida, H.; Takabayashi, A.; Kanai, M.; Adachi, M.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. Aminopeptidase N is involved in cell motility and angiogenesis: Its clinical significance in human colon cancer. Gastroenterology 2002, 122, 376–386. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, L.; Xie, Y.; Xu, W. NGR-based strategies for targeting delivery of chemotherapeutics to tumor vasculature. Anticancer Agents Med. Chem. 2012, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Curnis, F.; Longhi, R.; Crippa, L.; Cattaneo, A.; Dondossola, E.; Bachi, A.; Corti, A. Spontaneous formation of L-isoaspartate and gain of function in fibronectin. J. Biol. Chem. 2006, 281, 36466–36476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corti, A.; Curnis, F.; Arap, W.; Pasqualini, R. The neovasculature homing motif NGR: More than meets the eye. Blood 2008, 112, 2628–2635. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Yan, J.; Sharifi, J.; Bai, T.; Khawli, L.A.; Epstein, A.L. Comparison of three different targeted tissue factor fusion proteins for inducing tumor vessel thrombosis. Cancer Res. 2003, 63, 5046–5053. [Google Scholar]
- Liu, C.; Dickinson, C.; Shobe, J.; Doñate, F.; Ruf, W.; Edgington, T. A hybrid fibronectin motif protein as an integrin targeting selective tumor vascular thrombogen. Mol. Cancer Ther. 2004, 3, 793–801. [Google Scholar]
- Huang, Z.J.; Zhao, Y.; Luo, W.Y.; You, J.; Li, S.W.; Yi, W.C.; Wang, S.Y.; Yan, J.H.; Luo, Q. Targeting the vasculature of colorectal carcinoma with a fused protein of (RGD)(3)-tTF. ScientificWorldJournal 2013, 2013, 637086. [Google Scholar] [CrossRef]
- Kessler, T.; Bieker, R.; Padro, T.; Schwoppe, C.; Persigehl, T.; Bremer, C.; Kreuter, M.; Berdel, W.E.; Mesters, R.M. Inhibition of tumor growth by RGD peptide-directed delivery of truncated tissue factor to the tumor vasculature. Clin. Cancer Res. 2005, 11, 6317–6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, T.; Schwoppe, C.; Liersch, R.; Schliemann, C.; Hintelmann, H.; Bieker, R.; Berdel, W.E.; Mesters, R.M. Generation of fusion proteins for selective occlusion of tumor vessels. Curr. Drug Discov. Technol. 2008, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schwoppe, C.; Zerbst, C.; Frohlich, M.; Schliemann, C.; Kessler, T.; Liersch, R.; Overkamp, L.; Holtmeier, R.; Stypmann, J.; Dreiling, A.; et al. Anticancer therapy by tumor vessel infarction with polyethylene glycol conjugated retargeted tissue factor. J. Med. Chem. 2013, 56, 2337–2347. [Google Scholar] [CrossRef]
- Brand, C.; Frohlich, M.; Ring, J.; Schliemann, C.; Kessler, T.; Mantke, V.; Konig, S.; Lucke, M.; Mesters, R.M.; Berdel, W.E.; et al. Tumor Growth Inhibition via Occlusion of Tumor Vasculature Induced by N-Terminally PEGylated Retargeted Tissue Factor tTF-NGR. Mol. Pharm. 2015, 12, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Schwoppe, C.; Kessler, T.; Persigehl, T.; Liersch, R.; Hintelmann, H.; Dreischaluck, J.; Ring, J.; Bremer, C.; Heindel, W.; Mesters, R.M.; et al. Tissue-factor fusion proteins induce occlusion of tumor vessels. Thromb. Res. 2010, 125 (Suppl. 2), S143–S150. [Google Scholar] [CrossRef]
- Persigehl, T.; Ring, J.; Bremer, C.; Heindel, W.; Holtmeier, R.; Stypmann, J.; Claesener, M.; Hermann, S.; Schafers, M.; Zerbst, C.; et al. Non-invasive monitoring of tumor-vessel infarction by retargeted truncated tissue factor tTF-NGR using multi-modal imaging. Angiogenesis 2014, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Baumeier, A.; Brand, C.; Grau, M.; Angenendt, L.; Harrach, S.; Stalmann, U.; Schmidt, L.H.; Gosheger, G.; Hardes, J.; et al. Aminopeptidase N (CD13): Expression, Prognostic Impact, and Use as Therapeutic Target for Tissue Factor Induced Tumor Vascular Infarction in Soft Tissue Sarcoma. Transl. Oncol. 2018, 11, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.H.; Stucke-Ring, J.; Brand, C.; Schliemann, C.; Harrach, S.; Muley, T.; Herpel, E.; Kessler, T.; Mohr, M.; Gorlich, D.; et al. CD13 as target for tissue factor induced tumor vascular infarction in small cell lung cancer. Lung Cancer 2017, 113, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.; Geng, L.; Qu, S.; Scarfone, C.; Giorgio, T.; Donnelly, E.; Gao, X.; Clanton, J. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell 2003, 3, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Seidi, K.; Jahanban-Esfahlan, R.; Monhemi, H.; Zare, P.; Minofar, B.; Daei Farshchi Adli, A.; Farajzadeh, D.; Behzadi, R.; Mesgari Abbasi, M.; Neubauer, H.A.; et al. NGR (Asn-Gly-Arg)-targeted delivery of coagulase to tumor vasculature arrests cancer cell growth. Oncogene 2018, 37, 3967–3980. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Monhemi, H.; Adli, A.D.F.; Minofar, B.; Zare, P.; Farajzadeh, D.; Farajnia, S.; Behzadi, R.; Abbasi, M.M. RGD delivery of truncated coagulase to tumor vasculature affords local thrombotic activity to induce infarction of tumors in mice. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Johansson, S.; Svineng, G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-integrin interactions. Front. Biosci. 1997, 2, d126–d146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zardi, L.; Carnemolla, B.; Siri, A.; Petersen, T.; Paolella, G.; Sebastio, G.; Baralle, F. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987, 6, 2337–2342. [Google Scholar] [CrossRef]
- Castellani, P.; Viale, G.; Dorcaratto, A.; Nicolo, G.; Kaczmarek, J.; Querze, G.; Zardi, L. The fibronectin isoform containing the ED-B oncofetal domain: A marker of angiogenesis. Int. J. Cancer 1994, 59, 612–618. [Google Scholar] [CrossRef]
- Nilsson, F.; Kosmehl, H.; Zardi, L.; Neri, D. Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumors in mice. Cancer Res. 2001, 61, 711–716. [Google Scholar]
- Shi, Q.; Zhang, Y.; Liu, S.; Liu, G.; Xu, J.; Zhao, X.; Anderson, G.J.; Nie, G.; Li, S. Specific tissue factor delivery using a tumor-homing peptide for inducing tumor infarction. Biochem. Pharmacol. 2018, 156, 501–510. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ho, S.-H.; Li, B.; Wang, M.; Deng, X.; Yang, N.; Liu, G.; Lu, Z.; Xu, J. Combination of tumour-infarction therapy and chemotherapy via the co-delivery of doxorubicin and thrombin encapsulated in tumour-targeted nanoparticles. Nat. Biomed. Eng. 2020, 4, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tian, Y.; Zhao, Y.; Zhang, Y.; Su, S.; Wang, J.; Wu, M.; Shi, Q.; Anderson, G.J.; Thomsen, J.; et al. pHLIP-mediated targeting of truncated tissue factor to tumor vessels causes vascular occlusion and impairs tumor growth. Oncotarget 2015, 6, 23523–23532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Zhang, C.; Liu, Z.; Huang, Q.; Zhang, Y.; Li, S.; Nie, G.; Tang, H.; Wang, Y. Metabonomic Investigation of Biological Effects of a New Vessel Target Protein tTF-pHLIP in a Mouse Model. J. Proteome Res. 2019, 19, 238–247. [Google Scholar] [CrossRef]
- Brand, C.; Schliemann, C.; Ring, J.; Kessler, T.; Baumer, S.; Angenendt, L.; Mantke, V.; Ross, R.; Hintelmann, H.; Spieker, T.; et al. NG2 proteoglycan as a pericyte target for anticancer therapy by tumor vessel infarction with retargeted tissue factor. Oncotarget 2016, 7, 6774–6789. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, H.; Donate, F.; Dickinson, C.; Santucci, R.; El-Sheikh, A.; Vessella, R.; Edgington, T.S. Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res. 2002, 62, 5470–5475. [Google Scholar] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, H.; Zhou, H.; Song, X.; Yuan, S.; Luo, Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 2006, 107, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- McRobb, L.S.; Lee, V.S.; Simonian, M.; Zhao, Z.; Thomas, S.G.; Wiedmann, M.; Raj, J.V.; Grace, M.; Moutrie, V.; McKay, M.J.; et al. Radiosurgery alters the endothelial surface proteome: Externalized intracellular molecules as potential vascular targets in irradiated brain arteriovenous malformation. Radiat. Res. 2017, 187, 66–78. [Google Scholar] [CrossRef]
- McRobb, L.S.; McKay, M.J.; Gamble, J.R.; Grace, M.; Moutrie, V.; Santos, E.D.; Lee, V.S.; Zhao, Z.; Molloy, M.P.; Stoodley, M.A. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging (Albany NY) 2017, 9, 1248–1268. [Google Scholar] [CrossRef] [Green Version]
- McRobb, L.S.; McKay, M.J.; Gauden, A.J.; Lee, V.S.; Subramanian, S.; Thomas, S.G.; Wiedmann, M.K.; Moutrie, V.; Grace, M.; Zhao, Z.; et al. Radiation-Stimulated Translocation of CD166 and CRYAB to the Endothelial Surface Provides Potential Vascular Targets on Irradiated Brain Arteriovenous Malformations. Int. J. Mol. Sci. 2019, 20, 5830. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Zhao, Z.; Faqihi, F.; Grau, G.E.; Combes, V.; Inglis, D.W.; Moutrie, V.; Stoodley, M.A.; McRobb, L.S. Targeting of externalized alphaB-crystallin on irradiated endothelial cells with pro-thrombotic vascular targeting agents: Potential applications for brain arteriovenous malformations. Thromb. Res. 2020, 189, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gregorc, V.; Cavina, R.; Novello, S.; Grossi, F.; Lazzari, C.; Capelletto, E.; Genova, C.; Salini, G.; Lambiase, A.; Santoro, A. NGR-hTNF and Doxorubicin as Second-Line Treatment of Patients with Small Cell Lung Cancer. Oncologist 2018, 23, 1133-e112. [Google Scholar] [CrossRef] [Green Version]
- Bieker, R.; Kessler, T.; Schwöppe, C.; Padró, T.; Persigehl, T.; Bremer, C.; Dreischalück, J.; Kolkmeyer, A.; Heindel, W.; Mesters, R.M. Infarction of tumor vessels by NGR-peptide–directed targeting of tissue factor: Experimental results and first-in-man experience. Blood 2009, 113, 5019–5027. [Google Scholar] [CrossRef] [Green Version]
- Schliemann, C.; Gerwing, M.; Heinzow, H. First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study. Cancers (Basel) 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Li, S.; Thorpe, P.E. A simple and rapid method for purifying the extracellular domain of human tissue factor. Thromb. Res. 1998, 91, 249–253. [Google Scholar] [CrossRef]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [Green Version]
- Maynard, J.; Heckman, C.; Pitlick, F.; Nemerson, Y. Association of tissue factor activity with the surface of cultured cells. J. Clin. Investig. 1975, 55, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef]
- Gajsiewicz, J.M.; Morrissey, J.H. Structure-Function Relationship of the Interaction between Tissue Factor and Factor VIIa. J. Thromb. Haemost. 2015, 41, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.H.; Fair, D.S.; Edgington, T.S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb. Res. 1988, 52, 247–261. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Zarghami, N. Tumor vascular infarction: Prospects and challenges. Int. J. Hematol. 2017, 105, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Thoreau, F.; Vanwonterghem, L.; Henry, M.; Coll, J.L.; Boturyn, D. Design of RGD-ATWLPPR peptide conjugates for the dual targeting of alphaVbeta3 integrin and neuropilin-1. Org. Biomol. Chem. 2018, 16, 4101–4107. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lv, H.; Ye, M.; Wang, S.; Ni, E.; Zeng, F.; Cao, C.; Luo, F.; Yan, J. Novel superparamagnetic iron oxide nanoparticles for tumor embolization application: Preparation, characterization and double targeting. Int. J. Pharm. 2012, 426, 248–255. [Google Scholar] [CrossRef]
- Zou, M.; Xu, P.; Wang, L.; Wang, L.; Li, T.; Liu, C.; Shi, L.; Xie, J.; Li, W.; Wang, S.; et al. Design and construction of a magnetic targeting pro-coagulant protein for embolic therapy of solid tumors. Artif. Cells Nanomed. Biotechnol. 2020, 48, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zou, M.; Wang, S.; Li, T.; Liu, C.; Wang, L.; Wang, L.; Luo, F.; Wu, T.; Yan, J. Construction and characterization of a truncated tissue factorcoagulation based composite system for selective thrombosis in tumor blood vessels. Int. J. Oncol. 2019, 55, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Brand, C.; Dencks, S.; Schmitz, G.; Mühlmeister, M.; Stypmann, J.; Ross, R.; Hintelmann, H.; Schliemann, C.; Müller-Tidow, C.; Mesters, R.M.; et al. Low-Energy Ultrasound Treatment Improves Regional Tumor Vessel Infarction by Retargeted Tissue Factor. J. Ultrasound Med. 2015, 34, 1227–1236. [Google Scholar] [CrossRef]
- Brand, C.; Greve, B.; Bölling, T.; Eich, H.T.; Willich, N.; Harrach, S.; Hintelmann, H.; Lenz, G.; Mesters, R.M.; Kessler, T.; et al. Radiation synergizes with antitumor activity of CD13-targeted tissue factor in a HT1080 xenograft model of human soft tissue sarcoma. PLoS ONE 2020, 15, e0229271. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Park, J.H.; Lin, K.Y.; Singh, N.; Schwoppe, C.; Mesters, R.; Berdel, W.E.; Ruoslahti, E.; Sailor, M.J.; Bhatia, S.N. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011, 10, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Gu, H. Targeting nucleolin to obstruct vasculature feeding with an intelligent DNA nanorobot. J. Cell Mol. Med. 2019, 23, 2248–2250. [Google Scholar] [CrossRef] [Green Version]
- Mylonakis, E.; Calderwood, S.B. Infective endocarditis in adults. N. Engl. J. Med. 2001, 345, 1318–1330. [Google Scholar] [CrossRef]
- Hemker, H.C.; Bas, B.M.; Muller, A.D. Activation of a pro-enzyme by a stoichiometric reaction with another protein. The reaction between prothrombin and staphylocoagulase. Biochim. Biophys. Acta 1975, 379, 180–188. [Google Scholar] [CrossRef]
- Panizzi, P.; Friedrich, R.; Fuentes-Prior, P.; Bode, W.; Bock, P.E. The staphylocoagulase family of zymogen activator and adhesion proteins. Cell. Mol. Life Sci. 2004, 61, 2793–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, R.; Panizzi, P.; Fuentes-Prior, P.; Richter, K.; Verhamme, I.; Anderson, P.J.; Kawabata, S.; Huber, R.; Bode, W.; Bock, P.E. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature 2003, 425, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, P.; Friedrich, R.; Fuentes-Prior, P.; Richter, K.; Bock, P.E.; Bode, W. Fibrinogen substrate recognition by staphylocoagulase.(pro)thrombin complexes. J. Biol. Chem. 2006, 281, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.G.; McAdow, M.; Kim, H.K.; Bae, T.; Missiakas, D.M.; Schneewind, O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010, 6, e1001036. [Google Scholar] [CrossRef] [Green Version]
- Kastrup, C.J.; Boedicker, J.Q.; Pomerantsev, A.P.; Moayeri, M.; Bian, Y.; Pompano, R.R.; Kline, T.R.; Sylvestre, P.; Shen, F.; Leppla, S.H.; et al. Spatial localization of bacteria controls coagulation of human blood by ‘quorum acting’. Nat. Chem. Biol. 2008, 4, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Farkas, Á.Z.; Farkas, V.J.; Szabó, L.; Wacha, A.; Bóta, A.; Csehi, L.; Kolev, K.; Thelwell, C. Structure, Mechanical, and Lytic Stability of Fibrin and Plasma Coagulum Generated by Staphylocoagulase From Staphylococcus aureus. Front. Immunol. 2019, 10, 2967. [Google Scholar] [CrossRef] [PubMed]
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Mojovic, B.; Mojovic, N.; Tager, M.; Drummond, M.C. Staphylocoagulase as a hemostatic agent. Yale J. Biol. Med. 1969, 42, 11–20. [Google Scholar] [PubMed]
- Berdel, W.E.; Harrach, S.; Brand, C.; Brömmel, K.; Berdel, A.F.; Hintelmann, H.; Schliemann, C.; Schwöppe, C. Animal Safety, Toxicology, and Pharmacokinetic Studies According to the ICH S9 Guideline for a Novel Fusion Protein tTF-NGR Targeting Procoagulatory Activity into Tumor Vasculature: Are Results Predictive for Humans? Cancers (Basel) 2020, 12, 3536. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faqihi, F.; Stoodley, M.A.; McRobb, L.S. The Evolution of Safe and Effective Coaguligands for Vascular Targeting and Precision Thrombosis of Solid Tumors and Vascular Malformations. Biomedicines 2021, 9, 776. https://doi.org/10.3390/biomedicines9070776
Faqihi F, Stoodley MA, McRobb LS. The Evolution of Safe and Effective Coaguligands for Vascular Targeting and Precision Thrombosis of Solid Tumors and Vascular Malformations. Biomedicines. 2021; 9(7):776. https://doi.org/10.3390/biomedicines9070776
Chicago/Turabian StyleFaqihi, Fahimeh, Marcus A. Stoodley, and Lucinda S. McRobb. 2021. "The Evolution of Safe and Effective Coaguligands for Vascular Targeting and Precision Thrombosis of Solid Tumors and Vascular Malformations" Biomedicines 9, no. 7: 776. https://doi.org/10.3390/biomedicines9070776