Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin
Abstract
:1. Introduction
2. Chemistry of Umbelliprenin
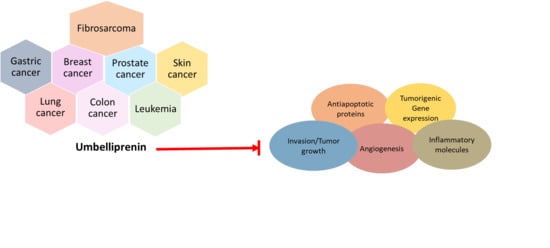
3. Role of Umbelliprenin (UMB) in Cancer and Its Molecular Targets
3.1. Breast Cancer
3.2. Lung Cancer
3.3. Colon Cancer
3.4. Gastric Cancer
3.5. Prostate Cancer
3.6. Skin Cancer
3.7. Leukemia
3.8. Fibrosarcoma
4. Strategies to Enhance UMB’s Bioavailability
4.1. Nanoparticles Drug Delivery
4.2. Liposomes
5. Hormesis Effect Caused by UMB
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UMB | Umbelliprenin |
| BAX | Bcl2-Associated X Protein |
| BCL-2 | B-Cell Lymphoma-2 |
| CDK | Cyclin Dependent Kinase |
| CRC | Colorectal Cancer |
| CRPC | Castration Resistance Prostate Cancer |
| EGFR | Epidermal Cell Growth Factor Receptor |
| EMT | Epithelial-Mesenchymal |
| IL-4 | Interleukin 4 |
| IL-10 | Interleukin-10 |
| MMP-9 | Matrix Metalloproteinase 9 |
| MMP-2 | Matrix Metalloproteinase 2 |
| NF-kB | Nuclear Factor Kappa Light Chain Enhancer of Activated B Cells |
| PARP | Poly ADP-Ribose Polymerase |
| VEGF | Vascular Endothelial Growth Factor |
| MDR | Multiple drug resistance |
| PLGA | Poly Lactic-Glycolic Acid |
| PLA | Poly Lactic Acid |
| TNF | Tumor necrosis factor |
| Foxp | Forkhead box protein |
| IFN | Interferone |
| 15-LOX | Lysyl oxidase |
| TGF | Transforming Growth Factor |
| µg | Microgram |
| µM | Micromole |
| CLL | chronic Lymphocytic Leukemia |
| Rb-4 | Retinoblastoma 4 |
| VHR | vaccinia H1-related |
| DDAH | dimethylarginine dimethylaminohydrolase |
| APRT | Adenine Phosphoribosyltransferase |
| MST | Macrophage-stimulating protein |
| SF3A3 | Splicing factor 3A subunit 3 |
References
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2018, 46, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aruoma, O.I.; Bahorun, T.; Agnihotri, A.K. Cancer risks and perspectives: Molecular mechanisms. Fundam. Mol. Mech. Mutagen. 2014, 768, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose–response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Neel, B.A.; Antoniou, I.M.; Yakar, S.; LeRoith, D. The increased risk of cancer in obesity and type 2 diabetes: Potential mechanisms. In Principles of Diabetes Mellitus, 3rd ed.; Springer International Publishing: Boston, MA, USA, 2017; pp. 731–753. ISBN 9783319187419. [Google Scholar]
- Leon, M.E.; Peruga, A.; McNeill, A.; Kralikova, E.; Guha, N.; Minozzi, S.; Espina, C.; Schüz, J. European Code against Cancer, 4th Edition: Tobacco and cancer. Cancer Epidemiol. 2015, 39, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Sagepub. 2017, 24, 1073274817729245. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sheng, S.; Margarida Bernardo, M.; Dzinic, S.H.; Chen, K.; Heath, E.I.; Sakr, W.A. Tackling tumor heterogeneity and phenotypic plasticity in cancer precision medicine: Our experience and a literature review. Cancer Metastasis Rev. 2018, 37, 655–663. [Google Scholar] [CrossRef]
- Devita, V.T.; Canellos, G.P. New therapies and standard of care in oncology. Nat. Rev. Clin. Oncol. 2011, 8, 67–68. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharm. 2017, 15, 503–519. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharm. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.V.G.K.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40, 48–81. [Google Scholar] [CrossRef] [PubMed]
- John, K. Borchardt the Beginnings of Drug Therapy: Ancient Mesopotamian Medicine. Drug News Perspect. 2002, 15, 187. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef]
- Patwardhan, B.; Gautam, M. Botanical immunodrugs: Scope and opportunities. Drug Discov. Today 2005, 10, 495–502. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [PubMed]
- Dilshad, E.; Ismail, H.; Khan, M.A.; Cusido, R.M.; Mirza, B. Metabolite profiling of Artemisia carvifolia Buch transgenic plants and estimation of their anticancer and antidiabetic potential. Biocatal. Agric. Biotechnol. 2020, 24, 101539. [Google Scholar] [CrossRef]
- Dilshad, E.; Zafar, S.; Ismail, H.; Waheed, M.T.; Cusido, R.M.; Palazon, J.; Mirza, B. Effect of Rol Genes on Polyphenols Biosynthesis in Artemisia annua and Their Effect on Antioxidant and Cytotoxic Potential of the Plant. Appl. Biochem. Biotechnol. 2016, 179, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Nagulapalli Venkata, K.C.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Potential of neem (Azadirachta indica) for prevention and treatment of oncologic diseases. Semin. Cancer Biol. 2016, 40, 100–115. [Google Scholar] [CrossRef]
- Di Fabio, G.; Romanucci, V.; Zarrelli, M.; Giordano, M.; Zarrelli, A. C-4 gem-dimethylated Oleanes of Gymnema sylvestre and their pharmacological activities. Molecules 2013, 18, 14892–14919. [Google Scholar] [CrossRef] [Green Version]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Natale, A.; Oriano, P.; Zarrelli, A. Structural characterization of phytotoxic terpenoids from Cestrum parqui. Phytochemistry 2005, 66, 2681–2688. [Google Scholar] [CrossRef]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Zarrelli, A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agric. Food Chem. 2004, 52, 4101–4108. [Google Scholar] [CrossRef]
- Wang, X.J.; Chen, J.Y.; Fu, L.Q.; Yan, M.J. Recent advances in natural therapeutic approaches for the treatment of cancer. J. Chemother. 2020, 32, 53–65. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinfl ammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. γ-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [Green Version]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [Green Version]
- Calcio, G.E.; Tagliapietra, S.; Martina, K.; Palmisano, G.; Cravotto, G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv. 2016, 6, 46394–46405. [Google Scholar] [CrossRef]
- Askari, M.; Sahebkar, A.; Iranshahi, M. Synthesis and Purification of 7-Prenyloxycoumarins and Herniarin as Bioactive Natural Coumarins. Iran. J. Basic Med. Sci. 2009, 12, 63–69. [Google Scholar]
- Iranshahi, M.; Shahverdi, A.R.; Mirjani, R.; Amin, G.; Shafiee, A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z. Nat. Sect. C J. Biosci. 2004, 59, 506–508. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Brodelius, P.E. Sesquiterpene coumarins. Phytochem. Rev. 2012, 11, 77–96. [Google Scholar] [CrossRef]
- Fiorito, S.; Genovese, S.; Palumbo, L.; Scotti, L.; Ciulla, M.; di Profio, P.; Epifano, F. Umbelliprenin as a novel component of the phytochemical pool from Artemisia spp. J. Pharm. Biomed. Anal. 2020, 184, 113205. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Natvar, P. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide, and nitric oxide free radical scavenging methods. J. Adv. Pharm. Res. 2011, 1, 52–68. [Google Scholar]
- Stanchev, S.; Momekov, G.; Jensen, F.; Manolov, I. Synthesis, computational study, and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008, 43, 694–706. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Davari, A.S.; Iranshahi, M.; Sabouri-Rad, S.; Tayarani Najaran, Z. Comparative analysis of the cytotoxic effect of 7-prenyloxycoumarin compounds and herniarin on MCF-7 cell line. Avicenna J. Phytome. 2015, 5, 520–530. [Google Scholar]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G. Prenylated Flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2010, 12, 713–739. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Lim, S.; Iranshahi, M.; Chollet, P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented malignant melanoma cells through cell-cycle arrest in G1 and induction of caspase-dependent apoptosis. Phytomedicine 2008, 15, 103–111. [Google Scholar] [CrossRef]
- Ziai, S.A.; Gholami, O.; Iranshahi, M.; Zamani, A.H.; Jeddi-Tehrani, M. Umbelliprenin induces apoptosis in CLL cell lines. Iran. J. Pharm. Res. 2012, 11, 653–659. [Google Scholar]
- Khaghanzadeh, N.; Mojtahedi, Z.; Ramezani, M.; Erfani, N.; Ghaderi, A. Umbelliprenin is cytotoxic against QU-DB large cell lung cancer cell line but anti-proliferative against A549 adenocarcinoma cells. DARU J. Pharm. Sci. 2012, 20, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranshahi, M.; Sahebkar, A.; Takasaki, M.; Konoshima, T.; Tokuda, H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur. J. Cancer Prev. 2009, 18, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Khaghanzadeh, N.; Samiei, A.; Ramezani, M.; Mojtahedi, Z.; Hosseinzadeh, M.; Ghaderi, A. Umbelliprenin induced production of IFN-γ and TNF-α, and reduced IL-10, IL-4, Foxp3 and TGF-β in a mouse model of lung cancer. Immunopharmacol. Immunotoxicol. 2014, 36, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kavetsou, E.; Gkionis, L.; Galani, G.; Gkolfinopoulou, C.; Argyri, L.; Pontiki, E.; Chroni, A.; Hadjipavlou-Litina, D.; Detsi, A. Synthesis of prenyloxy coumarin analogues and evaluation of their antioxidant, lipoxygenase (LOX) inhibitory and cytotoxic activity. Med. Chem. Res. 2017, 26, 856–866. [Google Scholar] [CrossRef]
- Saboormaleki, S.; Sadeghian, H.; Bahrami, A.R.; Orafaie, A.; Matin, M.M. 7-farnesyloxycoumarin exerts anti-cancer effects on a prostate cancer cell line by 15-LOX-1 inhibition. Arch. Iran. Med. 2018, 21, 251–259. [Google Scholar]
- Shahverdi, A.R.; Saadat, F.; Khorramizadeh, M.R.; Iranshahi, M.; Khoshayand, M.R. Two matrix metalloproteinases inhibitors from Ferula persica var. persica. Phytomedicine 2006, 13, 712–717. [Google Scholar] [CrossRef]
- Bayrami, G.; Boskabady, M.H.; Iranshahi, M.; Gholamnezhad, Z. Relaxant effects of asafoetida extract and its constituent umbelliprenin on guinea-pig tracheal smooth muscle. Chin. J. Integr. Med. 2013, 12, 1–6. [Google Scholar] [CrossRef]
- Tarver, T. American Cancer Society. Cancer facts and figures 2014. J. Consum. Health Internet 2012, 16, 366–367. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chiang, S.Y.; Nam, D.; Chung, W.S.; Lee, J.; Na, Y.S.; Sethi, G.; Ahn, K.S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Ziai, S.A.; Zanjani, T.M.; Khalilnezhad, A.; Jamshidi, H.; Amani, D. Umbelliprenin is potentially toxic against the HT29, CT26, MCF-7, 4T1, A172, and Gl26 cell lines, potentially harmful against bone marrow-derived stem cells, and non-toxic against peripheral blood mononuclear cells. Iran. Red Crescent Med. J. 2016, 18, e35167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, M.; Khalilnezhad, A.; Amani, D.; Jamshidi, H.; Muhammadnejad, A.; Bazi, A.; Ziai, S.A. Umbelliprenin shows antitumor, antiangiogenesis, antimetastatic, anti-inflammatory, and immunostimulatory activities in 4T1 tumor bearing Balb/c mice. J. Cell. Physiol. 2018, 233, 8908–8918. [Google Scholar] [CrossRef]
- Zhang, L.; Si, J.; Li, G.; Li, X.; Zhang, L.; Gao, L.; Huo, X.; Liu, D.; Sun, X. CaoUmbelliprenin and Lariciresinol isolated from a long-term-used herb medicine Ferula sinkiangensis induce apoptosis and G0/G1 arresting in gastric cancer cells. RSC Adv. 2015, 5, 91006–91017. [Google Scholar] [CrossRef]
- Valiahdi, S.M.; Iranshahi, M.; Sahebkar, A. Cytotoxic activities of phytochemicals from Ferula species. DARU J. Pharm. Sci. 2013, 21, 39. [Google Scholar] [CrossRef] [Green Version]
- Soltani, S.; Amin, G.; Salehi-Sourmaghi, M.H.; Iranshahi, M. Histone deacetylase inhibitory and cytotoxic activities of the constituents from the roots of three species of Ferula. Iran. J. Basic Med. Sci. 2019, 22, 93–98. [Google Scholar]
- Khaghanzadeh, N.; Nakamura, K.; Kuramitsu, Y.; Ghaderi, A.; Mojtahedi, Z. Immune-associated proteins with potential in vivo anti-tumor activities are upregulated in lung cancer cells treated with umbelliprenin: A proteomic approach. Oncol. Lett. 2016, 12, 5295–5302. [Google Scholar] [CrossRef] [Green Version]
- Hamidinia, M.; Ramezani, M.; Mojtahedi, Z. Cytotoxic/proliferative effects of umbelliprenin on colon cancer cell lines. Q. Ann. Color. Res. 2013, 1, 101–105. [Google Scholar] [CrossRef]
- Ooi, C.H.; Ivanova, T.; Wu, J.; Lee, M.; Tan, I.B.; Tao, J.; Ward, L.; Koo, J.H.; Gopalakrishnan, V.; Zhu, Y.; et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009, 5, e1000676. [Google Scholar] [CrossRef] [Green Version]
- Bohle, B.; Pera, M.; Pascual, M.; Alonso, S.; Mayol, X.; Salvado, M.; Schmidt, J.; Grande, L. Postoperative intra-abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery 2010, 147, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, F.M.; Gholami, O. Comparison of Umbelliprenin and Auraptene in Cytotoxic Effects and Myeloid Cell Leukemia Type-1 (Mcl-1) Gene Expression. Indian J. Pharm. Sci. 2016, 78, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Kitada, S.; Reed, J.C. MCL-1 promoter insertions dial-up aggressiveness of chronic leukemia. J. Natl. Cancer Inst. 2004, 96, 642–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholami, O.; Jeddi-Tehrani, M.; Iranshahi, M.; Zarnani, A.H.; Ziai, S.A. Umbelliprenin from Ferula szowitsiana activates both intrinsic and extrinsic pathways of apoptosis in jurkat T-CLL cell line. Iran. J. Pharm. Res. 2013, 12, 371–376. [Google Scholar] [PubMed]
- Ebrahimi, K.; Bagheri, R.; Keramati, M.R.; Rassouli, F.B. Investigating viability of human leukemia/lymphoma cells upon coadministration of umbelliprenin and radiotherapy Anti-metastatic potential of crocin on triple negative breast cancer in mice model. DNA Cell Biol. 2019, 7, 439. [Google Scholar]
- Alizadeh, M.N.; Rashidi, M.; Muhammadnejad, A.; Zanjani, T.M.; Ziai, S.A. Antitumor effects of umbelliprenin in a mouse model of colorectal cancer. Iran. J. Pharm. Res. 2018, 17, 976–985. [Google Scholar]
- de Santis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol. Prev. Biomark. 2015, 24, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- Roshandel, G.; Boreiri, M.; Sadjadi, A.; Malekzadeh, R. A diversity of cancer incidence and mortality in West Asian populations. Ann. Glob. Heal. 2014, 80, 346–357. [Google Scholar] [CrossRef]
- Shin, E.M.; Hay, H.S.; Lee, M.H.; Goh, J.N.; Tan, T.Z.; Sen, Y.P.; Lim, S.W.; Yousef, E.M.; Ong, H.T.; Kong, X.; et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J. Clin. Invest. 2014, 124, 3807–3824. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anticancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane triterpenoids in the prevention and therapy of breast cancer: Current evidence and future perspectives. Phytochem. Rev. 2014, 13, 793–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, C.; Lee, S.G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a steroidal glycoside abrogates STAT3 signaling cascade and exhibits anti-cancer activity by causing GSH/GSSG imbalance in lung carcinoma. Cancers 2018, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine attenuates tumor growth and deactivates STAT5 signaling in a lung cancer xenograft model. Cancers 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Mohan, C.D.; Basappa, S.; Rangappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Kumar, A.P.; Sethi, G.; Ahn, K.S.; et al. The IκB kinase inhibitor ACHP targets the STAT3 signaling pathway in human non-small cell lung carcinoma cells. Biomolecules 2019, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Ong, P.-S.; Wang, L.; Chia, D.M.-H.; Seah, J.Y.-X.; Kong, L.-R.; Thuya, W.-L.; Chinnathambi, A.; Lau, J.-Y.A.; Wong, A.L.-A.; Yong, W.-P.; et al. A novel combinatorial strategy using Seliciclib® and Belinostat® for eradication of non-small cell lung cancer via apoptosis induction and BID activation. Cancer Lett. 2016, 381, 49–57. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Z.; Shen, X.; Li, L.; Zhong, J.; Min, L.S.; Xu, L.; Li, H.; Zhang, J.; Dai, L. Serum lipidomics profiling to identify biomarkers for non-small cell lung cancer. Biomed Res. Int. 2018. [Google Scholar] [CrossRef]
- Blanchon, F.; Grivaux, M.; Asselain, B.; Lebas, F.-X.; Orlando, J.-P.; Piquet, J.; Zureik, M. 4-year mortality in patients with non-small-cell lung cancer: Development and validation of a prognostic index. Lancet Oncol. 2006, 7, 829–836. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef] [Green Version]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer. Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshaker, H.A.; Matalka, K.Z. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: The significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazuelo, M.B.; Correa, P. Gastric cancer: Overview. Gastrol. Clin. N. Am. 2013, 44, 192–201. [Google Scholar]
- Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; Sasako, M.; et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. J. Am. Med. Assoc. 2010, 303, 1729–1737. [Google Scholar]
- Wang, C.; Zhang, J.; Cai, M.; Zhu, Z.; Gu, W.; Yu, Y.; Zhang, X. DBGC: A database of human gastric cancer. PLoS ONE 2015, 10, e0142591. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon; IARC Publications: Lyon, France, 2013. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S. Small molecule antagonists of Tcf4/β-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer 2010, 126, 2426–2436. [Google Scholar]
- Zhang, L.; Sun, X.; Si, J.; Li, G.; Cao, L. Umbelliprenin isolated from Ferula sinkiangensis inhibits tumor growth and migration through the disturbance of Wnt signaling pathway in gastric cancer. PLoS ONE 2019, 14, e0207169. [Google Scholar] [CrossRef] [Green Version]
- Marberger, M.; Carroll, P.R.; Zelefsky, M.J.; Coleman, J.A.; Hricak, H.; Scardino, P.T.; Abenhaim, L.L. New treatments for localized prostate cancer. Urology 2008, 72, 36–43. [Google Scholar] [CrossRef]
- Zerbib, M.; Zelefsky, M.J.; Higano, C.S.; Carroll, P.R. Conventional treatments of localized prostate cancer. Urology 2008, 72, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, N.B.; Morrison, C.C.; Blasi, P.R.; Nguyen, M.; Shibuya, K.C.; Patnode, C.D. Behavioral counseling for skin cancer prevention: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018, 319, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199. [Google Scholar] [CrossRef] [Green Version]
- Rigel, D.S.; Friedman, R.J.; Kopf, A.W. The incidence of malignant melanoma in the United States: Issues as we approach the 21st century. J. Am. Acad. Derm. 1996, 34, 839–847. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.-W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.; Dinnes, J.; Chuchu, N.; Takwoingi, Y.; Bayliss, S.E.; Matin, R.N.; Jain, A.; Walter, F.M.; Williams, H.C.; Deeks, J.J. Algorithm based smartphone apps to assess risk of skin cancer in adults: Systematic review of diagnostic accuracy studies. BMJ 2020, 368, 127. [Google Scholar] [CrossRef] [Green Version]
- Dib, E.G. Chronic Lymphocytic Leukemia: Molecular Genetics, Biology, Diagnosis, and Management. Leuk. Res. 2004, 28, 995. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic lymphocytic leuke. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholami, O.; Jeddi-Tehrani, M.; Iranshahi, M.; Zarnani, A.H.; Ziai, S.A. Mcl-1 is up regulated by prenylated coumarin, umbelliprenin in Jurkat cells. Iran. J. Pharm. Res. 2014, 13, 1385–1390. [Google Scholar]
- Zhang, N.; Guo, L.; Kuang, H.; Ji, Y.; Zeng, X. Primary Intracranial Fibrosarcoma: Case Report and Systematic Review of Literature. World Neurosurg. 2019, 123, 251–255. [Google Scholar] [CrossRef]
- Teng, X.D. World Health Organization classification of tumours, pathology and genetics of tumours of the lung. Chin. J. Pathol. 2005, 34, 544–546. [Google Scholar]
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knösel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current diagnostics and treatment of fibrosarcoma-perspectives for future therapeutic targets and strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, E.I.; Grochow, L.B. Clinical potential of matrix metalloprotease inhibitors in cancer therapy. Drugs 2000, 59, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Iranshahi, M.; Hanafi-Bojd, M.Y.; Malaekeh-Nikouei, B. Preparation, characterization, and cytotoxic effects of nanoliposomes containing umbelliprenin. Int. J. Pharm. Res. 2014, 6, 79–84. [Google Scholar]
- Rashidi, M.; Ahmadzadeh, A.; Ziai, S.A.; Narenji, M.; Jamshidi, H. Evaluating cytotoxic effect of nanoliposomes encapsulated with umbelliprenin on 4T1 cell line. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.; Research, Y.C. Nanoparticles-a review. Trop. J. Pharm. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Miricescu, D.; Stanescu, I.; Perlea, P.; Calenic, B.; Radulescu, R.; Totan, A.; Virgolici, B.; Sabliov, C.; Greabu, M. Oxidative stress following PLGA nanoparticles administration to an animal model. Mater. Plast. 2017, 54, 249–252. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef]
- Khorramizadeh, M.R.; Esmail-Nazari, Z.; Zarei-Ghaane, Z.; Shakibaie, M.; Mollazadeh-Moghaddam, K.; Iranshahi, M.; Shahverdi, A.R. Umbelliprenin-coated Fe3O4 magnetite nanoparticles: Antiproliferation evaluation on human Fibrosarcoma cell line (HT-1080). Mater. Sci. Eng. C 2010, 30, 1038–1042. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.K.; Martin, F.J.; Jay, G.; Vogel, J.; Papahadjopoulos, D.; Friend, D.S. Extravasation, and transcytosis of liposomes in Kaposi’s sarcoma-like dermal lesions of transgenic mice bearing the HIV tat gene. Am. J. Pathol. 1993, 143, 10–14. [Google Scholar] [PubMed]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: Principles and applications. Homeopathy 2015, 104, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ. Pollut. 2005, 138, 378–411. [Google Scholar] [CrossRef] [PubMed]
- Gholami, O. Umbelliprenin mediates its apoptotic effect by hormesis: A commentary. Dose Response 2017, 15, 1559325817710035. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Cancer | Cell Line | Dose/IC 50 | Biological Effects | Molecular Targets | Reference |
|---|---|---|---|---|---|
| Breast cancer | MCF-7 4T1 mammary carcinoma cells | 73.4 µM,30.6 µg/mL, 59.7 µM 30.6–62.2 µg/mL for 24, 48, 72 h | Mitochondrial Apoptosis, rounding of cell, reduction of cell volume, DNA fragmentation, cell cycle arrest at G1 phase, Antiproliferative | ↑Bax | [48,51,65,66] |
| Lung cancer | M4Beu, A549, QU-DB | 52 µM, 133 µM 47 µM | Apoptosis, anti-proliferation, Anti-angiogenesis, Inhibit migration, Lymphocyte proliferation | ↓HSP90, ↓HSP27, ↓GRP94, ↓vimentin, ↓hnRNP C1/C2, ↓p97/VCP, ↓NDUFS3, ↓importin-α2, ↓importin-β1, ↓tubulin α-1B, ↓FKBP4, ↓SF3a3, ↓cyclophilin B, ↓APRT, ↓DDAH-2, ↓VHR, ↓annexin A4, ↓prohibitin, ↓proteasome α-1, ↓MST, ↓keratin-1, ↓heat shock protein 90 kDa, ↑Nipsnap1, ↑glycine-tRNA ligase, ↑stathmin (tumorigenic), ↑calreticulin | [51,53,67,68,69,70] |
| Colon cancer | DLD1, HT29 human CT26 mouse colon carcinoma SW48 cells SW1116 cells | 36.4 µg/mL 51.4 µg/mL 69 µM for 72 h | Reduced cell viability, Apoptosis | − | [51,65,71] |
| Gastric cancer | AGS, BGC-823, GES-1 | 11.74 µM, 24.62 µM | Mitochondrial apoptosis, ROS generation, increased, cycle arrest at G0/G1 phase, | ↑Bax, ↑caspase 3 expression, ↑p27, ↑P16, ↑Rb 4, ↓MMP9, ↓MMP2, ↓cyclin D, ↓cyclin E, ↓CDK4, ↓CDK2, ↓Wnt, ↓β-catenin, ↓Survivin, ↓c-myc, activation of PARP cleavage | [67,72,73] |
| Prostate cancer | PC3, DU145 | 4.3 µg/mL | Reduced cell viability, Apoptosis, DNA damage, cell cycle arrest at G1 phase, | ↓15-LOX expression | [51,58] |
| Skin cancer | SK-MEL-28 M4Beu | 58.1 µM 25 mM | Apoptosis, Antiproliferative, cell cycle arrest at G1 phase, | − | [68] |
| Leukemia | Jurkat T-CLL Raji B-CLL, MT2 cells, ATLL cell line | 11.3 µg/mL, 25 µM, 40 µg/mL + radiotherapy | Apoptosis, decrease cell viability | ↓Mcl-1 level, ↑caspase 3, caspase 9, caspase 8, ↑Bcl2 | [52,74,75,76,77] |
| Fibrosarcoma | Wehi 164 cells | 14 µg/mL | Apoptosis | ↓MMP | [59] |
| Esophageal carcinoma | KYSE-30 | 24.32 µg/mL | Reduced cell viability | − | [74] |
| Cervical cancer | HeLa | 20.22 µg/mL | Reduced cell viability | − | [69,74] |
| Ovarian cancer | CH1, A2780 | 37.2 µg/mL | Reduced cell viability | − | [51,68,69] |
| Glioma | GL26 mouse cancer cells A172 human cancer cells | 46.1 µg/mL 37.9 µg/mL For 48 h | Reduced cell viability | − | [65] |
| Cancer | Model | Phenotypic Effect | Molecular Target | References | |
|---|---|---|---|---|---|
| Breast Cancer | 4T1 tumor-bearing Balb/c mice | 200 µL of UMB (12.5 mg/mL) | Inhibit tumor growth, apoptosis, cell cycle arrest, anti-angiogenesis, ant-metastasis, anti-inflammatory, Th1 response | ↓VEGF, ↓COX2, ↓MMP2, ↓MMP9, NF-κB, ↓CD31, ↓CD31, ↓VCAM1, ↓IL-4 cytokines, ↑IFNγ ↑E-cadherin, ↑TNFR1 | |
| Lung cancer | LLC cells induction in mice | 2.5 mg/200 mL | Apoptosis, augmentation, cell mediated immune response | ↓IL-4, ↓IL-10, ↑TNF-a, ↑IFN-g, ↓Foxp3, ↓TGF-b | [55] |
| Colon cancer | CT26 cell line injected in Mice | Apoptosis, reduction of tumor size, angiogenesis, antiproliferative anti-metastasis, immune response | ↓VEGF, ↓MMP2, ↓MMP9, ↓CD31, ↓Ki-67, ↓MMP2, ↓MMP9, ↓IL-4 decrease, ↑E-cadherin, ↑IFN-γ | [78] | |
| Gastric cancer | human gastric cancer BGC-823 xenograft models | 43.33% (20 mg/kg) | Reduction in tumor volume, decrease in rats body weight | − | [67] |
| Skin cancer | vivo two-stage mouse skin | Less papillomas | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines 2020, 8, 126. https://doi.org/10.3390/biomedicines8050126
Shahzadi I, Ali Z, Baek SH, Mirza B, Ahn KS. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines. 2020; 8(5):126. https://doi.org/10.3390/biomedicines8050126
Chicago/Turabian StyleShahzadi, Iram, Zain Ali, Seung Ho Baek, Bushra Mirza, and Kwang Seok Ahn. 2020. "Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin" Biomedicines 8, no. 5: 126. https://doi.org/10.3390/biomedicines8050126