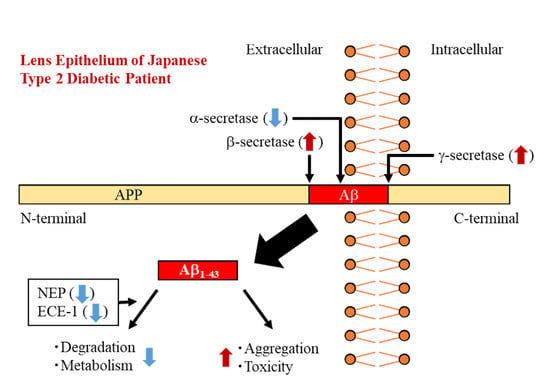
Enhancement of Amyloid β1–43 Production in the Lens Epithelium of Japanese Type 2 Diabetic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Collection of Lens Epithelium Samples
2.3. Measurement of Aβ-Producing and -Degrading Enzyme mRNAs by a Quantitative Real-Time RT-PCR Method
2.4. Measurement of Aβ1–40, Aβ1–42, and Aβ1–43 by ELISA Methods
2.5. Statistical Analysis
3. Results
3.1. Accumulation of Aβ in the Lens Epithelium of Diabetic Patients
3.2. Changes in Levels of Aβ-Producing and –Degrading Enzyme mRNAs in the Lens Epithelium of Diabetic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADAM10 | a disintegrin and metalloprotease domain protease 10 |
| APP | amyloid precursor protein |
| Aβ | amyloid β-protein |
| BACE1 | β site APP cleaving enzyme |
| DM | diabetes mellitus |
| ECE | endothelin converting enzyme |
| IDE | insulin-degrading enzyme |
| MMP-9 | matrix metalloproteinase 9 |
| NEP | neprilysin |
| NUC | nuclear opacification |
| OLETF rat | Otsuka Long-Evans Tokushima Fatty rat |
| PS | presenilin |
| PSC | posterior subcapsular opacification |
| RD | retrodots |
| sAPPα | soluble secretory APP |
| STZ rat | streptozotocin-induced diabetic rat |
| WC | water clefts |
| r | correlation coefficient |
References
- Gandy, S. The role of cerebral amyloid β accumulation in common forms of Alzheimer disease. J. Clin. Investig. 2005, 115, 1121–1129. [Google Scholar]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [Green Version]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, W.H.; Gleeson, P.A. Dysregulation of intracellular trafficking and endosomal sorting in Alzheimer’s disease: Controversies and unanswered questions. Biochem. J. 2016, 473, 1977–1993. [Google Scholar] [CrossRef] [PubMed]
- Bitan, G.; Kirkitadze, M.D.; Lomakin, A.; Vollers, S.S.; Benedek, G.B.; Teplow, D.B. Amyloid β-protein (Aβ) assembly: Aβ 40 and Aβ 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 2002, 100, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Kirkitadze, M.D.; Kowalska, A. Molecular mechanisms initiating amyloid b-fibril formation in Alzheimer’s disease. Acta Biochim. Pol. 2005, 52, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T., Jr. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Suemoto, T.; Brouwers, N.; Sleegers, K.; Funamoto, S.; Mihira, N.; Matsuba, Y.; Yamada, K.; Nilsson, P.; Takano, J.; et al. Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci. 2011, 14, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conicella, A.E.; Fawzi, N.L. The C-terminal threonine of Abeta43 nucleates toxic aggregation via structural and dynamical changes in monomers and protofibrils. Biochemistry 2014, 53, 3095–3105. [Google Scholar] [CrossRef]
- Li, G.; Percontino, L.; Sun, Q.; Qazi, A.S.; Frederikse, P.H. Betaamyloid secretases and beta-amloid degrading enzyme expression in lens. Mol. Vis. 2003, 9, 179–183. [Google Scholar] [PubMed]
- Frederikse, P.H.; Zigler, J.S., Jr. Presenilin expression in the ocular lens. Curr. Eye Res. 1998, 17, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.E.; Muffat, J.A.; Cherny, R.A.; Moir, R.D.; Ericsson, M.H.; Huang, X.; Mavros, C.; Coccia, J.A.; Faget, K.Y.; Fitch, K.A.; et al. Cytosolic betaamyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet 2003, 361, 1258–1265. [Google Scholar] [CrossRef]
- Nagai, N.; Mano, Y.; Otake, H.; Shibata, T.; Kubo, E.; Sasaki, H. Amyloid β1-43 Accumulates in the Lens Epithelium of Cortical Opacification in Japanese Patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3294–3302. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ito, Y.; Shibata, T.; Kubo, E.; Sasaki, H. A positive feedback loop between nitric oxide and amyloid β (1-42) accelerates mitochondrial damage in human lens epithelial cells. Toxicology 2017, 381, 19–30. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Sasaki, S. Hyperglycemia Enhances the Production of Amyloid β1–42 in the Lenses of Otsuka Long-Evans Tokushima Fatty Rats, a Model of Human Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1408–1417. [Google Scholar] [CrossRef]
- Ristow, M. Neurodegenerative disorders associated with diabetes mellitus. J. Mol. Med. 2004, 82, 510–529. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Murata, S.; Katsukura, Y.; Suzuki, H.; Funaki, M.; Tachikawa, M.; Terasaki, T. Involvement of insulin degrading enzyme in insulin- and atrial natriuretic peptide sensitive internalization of amyloid-β peptide in mouse brain capillary endothelial cells. J. Alzheimers Dis. 2014, 38, 185–200. [Google Scholar] [CrossRef]
- Cheng, D.; Noble, J.; Tang, M.X.; Schupf, N.; Mayeux, R.; Luchsinger, J.A. Type 2 diabetes and late-onset Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2011, 31, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Thylefors, B.; Chylack, L.T., Jr.; Konyama, K.; Sasaki, K.; Sperduto, R.; Taylor, H.R.; West, S. WHO Cataract Grading Group. A simplified cataract grading system. Ophthalmic. Epidemiol. 2002, 9, 83–95. [Google Scholar] [CrossRef]
- Harding, J.J. Conformational changes in human lens proteins in cataract. Biochem. J. 1972, 129, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, C.R.; Schwab, I.R. Epidemiology of cataract-a major cause of preventable blindness. Bull. World Health Organ. 1981, 59, 493–501. [Google Scholar] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalivaeva, N.N.; Turner, A.J. The amyloid precursor protein: A biochemical enigma in brain development, function and disease. FEBS Lett. 2013, 587, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- Kepp, K.P. Alzheimer’s disease due to loss of function: A new synthesis of the available data. Prog. Neurobiol. 2016, 143, 36–60. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.J.; Tanzawa, K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997, 11, 355–364. [Google Scholar] [CrossRef]
- Turner, A.J.; Brown, C.D.; Carson, J.A.; Barnes, K. The neprilysin family in health and disease. Adv. Exp. Med. Biol. 2000, 477, 229–240. [Google Scholar]
- Turner, A.J.; Isaac, R.E.; Coates, D. The neprilysin (NEP) family of zinc metalloendopeptidases: Genomics and function. Bioessays 2001, 23, 261–269. [Google Scholar] [CrossRef]
- Hersh, L.B.; Rodgers, D.W. Neprilysin and amyloid beta peptide degradation. Curr. Alzheimer Res. 2008, 5, 225–231. [Google Scholar] [CrossRef]
- Turner, A.J.; Murphy, L.J. Molecular pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 1996, 51, 91–102. [Google Scholar] [CrossRef]
- Arbin, V.; Claperon, N.; Fournie-Zaluski, M.C.; Roques, B.P.; Peyroux, J. Effects of dual angiotensinconverting enzyme and neutral endopeptidase 24-11 chronic inhibition by mixanpril on insulin sensitivity in lean and obese Zucker rats. J. Cardiovasc. Pharmacol. 2003, 41, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Arbin, V.; Claperon, N.; Fournie-Zaluski, M.C.; Roques, B.P.; Peyroux, J. Acute effect of the dual angiotensin-converting enzyme and neutral endopeptidase 24-11 inhibitor mixanpril on insulin sensitivity in obese Zucker rat. Br. J. Pharmacol. 2001, 133, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Leung, N.; Lapointe, N.; Szeto, L.; Uffelman, K.D.; Giacca, A.; Rouleau, J.L.; Lewis, G.F. Vasopeptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats: Studies comparing a vasopeptidase inhibitor, angiotensin-converting enzyme inhibitor, and angiotensin II type I receptor blocker. Circulation 2003, 107, 1923–1929. [Google Scholar] [PubMed]
- Liu, Y.; Liu, L.; Lu, S.; Wang, D.; Liu, X.; Xie, L.; Wang, G. Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats. J. Endocrinol. Investig. 2011, 34, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hejtmancik, J.F.; Riazuddin, S.A.; McGreal, R.; Liu, W.; Cvekl, A.; Shiels, A. Chapter Eleven: Lens Biology and Biochemistry. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar]
- Nagai, N.; Ito, Y. Excessive hydrogen peroxide enhances the attachment of amyloid β1–42 in the lens epithelium of UPL rats, a hereditary model for cataracts. Toxicology 2014, 315, 55–64. [Google Scholar] [CrossRef]
- Nagai, N.; Mano, Y.; Otake, H.; Shibata, T.; Kubo, E.; Sasaki, H. Changes in mitochondrial cytochrome c oxidase mRNA levels with cataract severity in lens epithelia of Japanese patients. Mol. Med. Rep. 2019, 19, 5464–5472. [Google Scholar] [CrossRef]
- Xu, J.; Li, D.; Zheng, T.; Lu, Y. β-amyloid expression in age-related cataract lens epithelia and the effect of β-amyloid on oxidative damage in human lens epithelial cells. Mol. Vis. 2017, 23, 1015–1028. [Google Scholar]
- Miners, J.S.; Barua, N.; Kehoe, P.G.; Gill, S.; Love, S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2011, 70, 944–959. [Google Scholar] [CrossRef] [Green Version]





| Characteristic | ELISA Method | PCR Method | ||||
|---|---|---|---|---|---|---|
| Clear | Opaque | Opaque + DM | Clear | Opaque | Opaque + DM | |
| Age (y) | 62.2 ± 14.9 | 73.9 ± 9.9 | 74.2 ± 7.8 | 64.1 ± 15.9 | 68.8 ± 13.8 | 69.3 ± 6.6 |
| Glucose (mg/dL) | 107.5 ± 16.3 | 102.3 ± 18.6 | 174.6 ± 72.6 | 97.7 ± 19.9 | 111.9 ± 41.9 | 247.3 ± 42.0 |
| Total opacification | 0 | 3.2 ± 1.6 | 3.2 ± 0.9 | 0 | 3.2 ± 1.5 | 6.0 ± 1.3 |
| Cortical opacification | 0 | 0 | 0 | 0 | 0 | 0 |
| Nuclear opacification | 0 | 1.0 ± 0.7 | 0.2 ± 0.3 | 0 | 0.8 ± 0.6 | 0.7 ± 0.3 |
| Posterior subcapsular opacification | 0 | 0.5 ± 1.0 | 0.6 ± 0.5 | 0 | 1.0 ± 1.0 | 0.3 ± 0.4 |
| Retrodots | 0 | 1.2 ± 1.4 | 1.8 ± 0.7 | 0 | 0.5 ± 0.6 | 3.0 ± 0.0 |
| Water clefts | 0 | 0.4 ± 0.6 | 0.6 ± 0.9 | 0 | 0.9 ± 1.0 | 2.0 ± 1.3 |
| n (60 samples) | Male 6, Female 7 | Male 7, Female 11 | Male 3, Female 2 | Male 3, Female 3 | Male 8, Female 7 | Male 2, Female 1 |
| Primer | Sequence (5′-3′) | |
|---|---|---|
| APP | FOR | TGGTGGGCGGTGTTGTCATA |
| REV | TGGATTTTCGTAGCCGTTCTGC | |
| BACE1 | FOR | GCAAGGAGTACAACTATGAC |
| REV | AGCTTCAAACACTTTCTTGG | |
| PS1 | FOR | ATCATCTGCATAGTCCTCTC |
| REV | AGACAGCTTTGATGTTCAAG | |
| ADAM10 | FOR | CACATGATTCTGGAACAGAG |
| REV | GTTGTTAAGTTTGTCCCCAG | |
| NEP | FOR | CTGATATCAACACTCCAAAGC |
| REV | TCATCGTAGGTTGCATAGAG | |
| ECE-1 | FOR | AGAATGAGATTGTGTTTCCG |
| REV | CTATGCCACCAAAGTTTAAGG | |
| β-actin | FOR | GTGGCATCCACGAAACTACC |
| REV | CAGGGCAGTGATCTCCTTCT |
| vs. Glucose | Approximation Formula | r |
|---|---|---|
| Total-Aβ levels | y = −1.3 × 10−2x + 11.5 | 0.089 |
| Aβ1–40 levels | y = 4.5 × 10−3x + 3.5 | 0.051 |
| Aβ1–42 levels | y = −2.2 × 10−2x + 8.4 | 0.225 |
| Aβ1–43 levels | y = 5.3 × 10−3x – 0.4 | 0.579 * |
| APP mRNA | y = 3.6 × 10−2x – 1.8 | 0.798 * |
| BACE1 mRNA | y = 0.2 × 10−5x – 0.2 × 10−3 | 0.419 * |
| PS1 mRNA | y = 0.2 × 10−5x – 0.1 × 10−4 | 0.320 |
| ADAM10 mRNA | y = −0.2 × 10−5x + 0.2 × 10−3 | 0.049 |
| NEP mRNA | y = −0.2 × 10−7x + 0.3 × 10−4 | 0.046 |
| ECE1 mRNA | y = −0.5 × 10−5x + 0.5 × 10−4 | 0.242 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano, Y.; Otake, H.; Shibata, T.; Kubo, E.; Sasaki, H.; Nagai, N. Enhancement of Amyloid β1–43 Production in the Lens Epithelium of Japanese Type 2 Diabetic Patients. Biomedicines 2020, 8, 87. https://doi.org/10.3390/biomedicines8040087
Mano Y, Otake H, Shibata T, Kubo E, Sasaki H, Nagai N. Enhancement of Amyloid β1–43 Production in the Lens Epithelium of Japanese Type 2 Diabetic Patients. Biomedicines. 2020; 8(4):87. https://doi.org/10.3390/biomedicines8040087
Chicago/Turabian StyleMano, Yu, Hiroko Otake, Teppei Shibata, Eri Kubo, Hiroshi Sasaki, and Noriaki Nagai. 2020. "Enhancement of Amyloid β1–43 Production in the Lens Epithelium of Japanese Type 2 Diabetic Patients" Biomedicines 8, no. 4: 87. https://doi.org/10.3390/biomedicines8040087