The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: A Systematic Review
Abstract
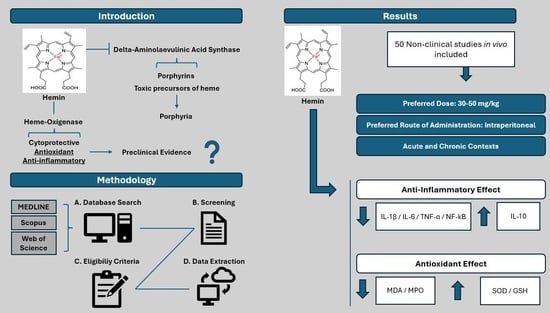
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process
2.5. Data Items
2.5.1. Population
2.5.2. Intervention
2.5.3. Comparator
2.5.4. Outcomes
2.5.5. Study Design
2.6. Quality and Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
4. Discussion
4.1. Hemin-Related Parameters
4.1.1. Dose
4.1.2. Frequency of Administration and Duration of Treatment
4.1.3. Route of Administration
4.2. Animal-Related Parameters
4.2.1. Disease Animal Model
4.2.2. Species and Strain
4.2.3. Gender
4.2.4. Age
4.3. Biomarkers Assessed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonkowsky, H.L.; Tschudy, D.P.; Collins, A.; Doherty, J.; Bossenmaier, I.; Cardinal, R.; Watson, C.J. Repression of the Overproduction of Porphyrin Precursors in Acute Intermittent Porphyria by Intravenous Infusions of Hematin. Proc. Natl. Acad. Sci. USA 1971, 68, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Collins, S. Open-Label Study of Hemin for Acute Porphyria: Clinical Practice Implication. Am. J. Med. 2006, 119, 801.e1–801.e6. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Stölzel, U.; Doss, M.O.; Schuppan, D. Clinical Guide and Update on Porphyrias. Gastroenterology 2019, 157, 365–381.e4. [Google Scholar] [CrossRef] [PubMed]
- Gasson, T.; Klein, K. Porphyria. Nurse Pract. 2015, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tahoun, M.; Engeser, M.; Svolacchia, L.; Sander, P.M.; Müller, C.E. Molecular Taphonomy of Heme: Chemical Degradation of Hemin under Presumed Fossilization Conditions. Molecules 2023, 28, 4887. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Hemin reduces inflammation associated with TNBS-induced colitis. Clin. Exp. Gastroenterol. 2018, 11, 325–334. [Google Scholar] [CrossRef]
- Silva, I.; Correia, R.; Pinto, R.; Mateus, V. Hemin Ameliorates the Inflammatory Activity in the Inflammatory Bowel Disease: A Non-Clinical Study in Rodents. Biomedicines 2022, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Uchiyama, K.; Yoshikawa, T. Heme oxygenase-1: A novel therapeutic target for gastrointestinal diseases. J. Clin. Biochem. Nutr. 2011, 48, 126–133. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Mimura, J.; Ozaki, T.; Itoh, K. Emerging Regulatory Role of Nrf2 in Iron, Heme, and Hemoglobin Metabolism in Physiology and Disease. Front. Vet. Sci. 2018, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Ivaldo, C.; Traverso, N.; Furfaro, A.L. Clinical Significance of Heme Oxygenase 1 in Tumor Progression. Antioxidants 2021, 10, 789. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Estarreja, J.; Caldeira, G.; Silva, I.; Mendes, P.; Mateus, V. The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: Protocol for a Systematic Review. JMIR Res. Protoc. 2023, 12, e48368. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Fan, G.; Zhao, H.; Li, J. Heme oxygenase-1 attenuates inflammation and oxidative damage in a rat model of smoke-induced emphysema. Int. J. Mol. Med. 2015, 36, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ren, W.; Zhang, Q.; Fu, N.; Han, F.; Cui, P.; Li, W.; Kong, L.; Zhao, S.; Wang, R.; et al. Heme Oxygenase-1 Suppresses Wnt Signaling Pathway in Nonalcoholic Steatohepatitis-Related Liver Fibrosis. BioMed Res. Int. 2020, 2020, 4910601. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Mi, H.-M.; Li, H.; Zhao, S.-X.; Jia, Y.-H.; Nan, Y.-M. Modulation of IKKβ/NF-κB and TGF-β1/Smad via Fuzheng Huayu recipe involves in prevention of nutritional steatohepatitis and fibrosis in mice. Iran. J. Basic Med. Sci. 2015, 18, 404–411. [Google Scholar]
- Tang, J.; Li, L.; Li, C.-M.; Wu, J.; Sun, Y.; Wang, G.-L. Upregulation of HO-1 with Haemin Alleviates LPS-Stimulated Pro-inflammatory Responses Through Downregulation of p38 Signalling Pathways in Rat Liver. Scand. J. Immunol. 2015, 82, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, J.; Sun, Y.; Wu, J.; Yu, P.; Wang, G. Upregulation of HO-1 Attenuates LPS-Stimulated Proinflammatory Responses Through Downregulation of p38 Signaling Pathways in Rat Ovary. Inflammation 2015, 38, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Al-Ashmawy, G.M.; Farag, A.A.; Ibrahim, A.O. Hemin versus erythropoietin: Possible role in Nrf2/HO-1 signaling pathway in rats with nephrotoxicity. Biomed. Pharmacother. 2022, 156, 113971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, W.; Ren, H.-Z.; Zhao, X.; Wang, S.; Ma, H.-C.; Shi, X.-L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Heeba, G.H.; Hamza, A.A.; Hassanin, S.O. Induction of heme oxygenase-1 with hemin alleviates cisplatin-induced reproductive toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Toxicol. Lett. 2016, 264, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.; Heeba, G.H.; El-Sheikh, A.A.K. Modulation of heme oxygenase-1 expression and activity affects streptozotocin-induced diabetic nephropathy in rats. Fundam. Clin. Pharmacol. 2017, 31, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Lv, J.J.; Lv, J.; Di, C.X.; Zhang, Y.J.; Zhou, T.; Liu, J.L.; Xia, Z.W. Heme oxygenase-1 directly binds STAT 3 to control the generation of pathogenic Th17 cells during neutrophilic airway inflammation. Allergy 2017, 72, 1972–1987. [Google Scholar] [CrossRef]
- Lin, X.; Lv, J.; Ge, D.; Bai, H.; Yang, Y.; Wu, J. Heme oxygenase-1 alleviates eosinophilic inflammation by inhibiting STAT3-SOCS3 signaling. Pediatr. Pulmonol. 2020, 55, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Di, C.; Lv, J.; Zhang, Y.; Lin, X.; Yuan, Y.; Lv, J.; Xia, Z. Heme oxygenase-1 inhibits basophil maturation and activation but promotes its apoptosis in T helper type 2-mediated allergic airway inflammation. Immunology 2016, 147, 321–337. [Google Scholar] [CrossRef]
- Konrad, F.M.; Zwergel, C.; Ngamsri, K.-C.; Reutershan, J. Anti-inflammatory Effects of Heme Oxygenase-1 Depend on Adenosine A2A- and A2B-Receptor Signaling in Acute Pulmonary Inflammation. Front. Immunol. 2017, 8, 1874. [Google Scholar] [CrossRef]
- Konrad, F.M.; Knausberg, U.; Höne, R.; Ngamsri, K.-C.; Reutershan, J. Tissue heme oxygenase-1 exerts anti-inflammatory effects on LPS-induced pulmonary inflammation. Mucosal Immunol. 2016, 9, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, Y.-H.; Fan, K.-L.; Dong, X.-B.; Han, N.; Zhao, H.; Kong, L. Protective effects of heme oxygenase-1 against severe acute pancreatitis via inhibition of tumor necrosis factor-α and augmentation of interleukin-10. BMC Gastroenterol. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-L.; Li, M.-F.; Chen, Y.-G. Effects of heme oxygenase-1 on cytokines and histological changes of pancreas and liver in rats with severe acute pancreatitis. Int. J. Clin. Exp. Pathol. 2016, 9, 7156–7163. [Google Scholar]
- Iwata, M.; Inoue, T.; Asai, Y.; Hori, K.; Fujiwara, M.; Matsuo, S.; Tsuchida, W.; Suzuki, S. The protective role of localized nitric oxide production during inflammation may be mediated by the heme oxygenase-1/20carbon monoxide pathway. Biochem. Biophys. Rep. 2020, 23, 100790. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Han, L.; Guo, L.; Zhong, H.; Wang, J. Hemin ameliorates influenza pneumonia by attenuating lung injury and regulating the immune response. Int. J. Antimicrob. Agents 2017, 49, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chaves, H.V.V.; do Val, D.R.; Ribeiro, K.A.; Lemos, J.C.; Souza, R.B.; Gomes, F.I.F.; da Cunha, R.M.S.; Pinto, V.P.T.; Filho, G.C.; de Souza, M.H.L.P.; et al. Heme oxygenase-1/biliverdin/carbon monoxide pathway downregulates hypernociception in rats by a mechanism dependent on cGMP/ATP-sensitive K+ channels. Inflamm. Res. 2018, 67, 407–422. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Ginter, G.; Pajdo, R.; Chmura, A.; Kwiecien, S.; Brzozowski, T. Carbon monoxide released from its pharmacological donor, tricarbonyldichlororuthenium (II) dimer, accelerates the healing of pre-existing gastric ulcers. Br. J. Pharmacol. 2017, 174, 3654–3668. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Lu, Y.; Zhao, L.; Xia, W.; Zhang, H.; Wang, L.; Zhang, L.; Wen, A. Hemin impairs resolution of inflammation via microRNA-144-3p-dependent downregulation of ALX/FPR2. Transfusion 2019, 59, 196–206. [Google Scholar] [CrossRef]
- Rossi, M.; Delbauve, S.; Roumeguère, T.; Wespes, E.; Leo, O.; Flamand, V.; Moine, A.L.; Hougardy, J.-M. HO-1 mitigates acute kidney injury and subsequent kidney-lung cross-talk. Free Radic. Res. 2019, 53, 1035–1043. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Zhao, H.; Qin, H.; Zhang, J.; Dong, J.; Zhang, H.; Liu, X.; Zhao, Z.; Zhao, Y.; et al. Carbon Monoxide Inhibits the Expression of Proteins Associated with Intestinal Mucosal Pyroptosis in a Rat Model of Sepsis Induced by Cecal Ligation and Puncture. Med. Sci. Monit. 2020, 26, e920668. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, H.-S.; Yim, S.-Y.; Lee, S.-M. Heme Oxygenase-1 Protects the Liver from Septic Injury by Modulating TLR4-Mediated Mitochondrial Quality Control in Mice. Shock 2018, 50, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wedn, A.M.; El-Gowilly, S.M.; El-Mas, M.M. The α7-nAChR/heme oxygenase-1/carbon monoxide pathway mediates the nicotine counteraction of renal inflammation and vasoconstrictor hyporeactivity in endotoxic male rats. Inflamm. Res. 2020, 69, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jamwal, S.; Bijjem, K.R.V.; Prakash, A.; Kumar, P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience 2015, 287, 66–77. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, B.; Zhang, Z.; Lu, H.; Fan, C.; Qi, Q.; Gao, Y.; Li, H.; Feng, C.; Zuo, J.; et al. Heme protects intestinal mucosal barrier in DSS-induced colitis through regulating macrophage polarization in both HO-1-dependent and HO-1-independent way. FASEB J. 2020, 34, 8028–8043. [Google Scholar] [CrossRef]
- Aycan-Ustyol, E.; Kabasakal, M.; Bekpinar, S.; Alp-Yıldırım, F.I.; Tepe, O.; Giris, M.; Ozluk, Y.; Unlucerci, Y.; Uydes-Dogan, B.S.; Uysal, M. Vascular function and arginine and dimethylarginines in gentamicin-induced renal failure: A possible effect of heme oxygenase 1 inducer hemin. Can. J. Physiol. Pharmacol. 2017, 95, 1406–1413. [Google Scholar] [CrossRef]
- Refaie, M.M.M.; Rifaai, R.A.; Bayoumi, A.M.A.; Shehata, S. Cardioprotective effect of hemin in isoprenaline-induced myocardial infarction: Role of ATP-sensitive potassium channel and endothelial nitric oxide synthase. Fundam. Clin. Pharmacol. 2020, 34, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.F.; Abdelzaher, W.Y.; Ibrahim, R.A.; Elroby Ali, D.M. Amelioration of estrogen-induced endometrial hyperplasia in female rats by hemin via heme-oxygenase-1 expression, suppression of iNOS, p38 MAPK, and Ki67. Can. J. Physiol. Pharmacol. 2019, 97, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Bayo Jimenez, M.T.; Frenis, K.; Kröller-Schön, S.; Kuntic, M.; Stamm, P.; Kvandová, M.; Oelze, M.; Li, H.; Steven, S.; Münzel, T.; et al. Noise-Induced Vascular Dysfunction, Oxidative Stress, and Inflammation Are Improved by Pharmacological Modulation of the NRF2/HO-1 Axis. Antioxidants 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, X.; Yu, J.; Cheng, X.; Ciao, Y.; Tang, F.; Li, Y.; Wan, S.; Su, W.; Liang, D. Hemin Promotes Corneal Allograft Survival Through the Suppression of Macrophage Recruitment and Activation. Investig. Opthalmology Vis. Sci. 2018, 59, 3952–3962. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Tiwari, S. Featured Article: Induction of heme oxygenase with hemin improves pericardial adipocyte morphology and function in obese Zucker rats by enhancing proteins of regeneration. Exp. Biol. Med. 2015, 240, 45–57. [Google Scholar] [CrossRef]
- Cheng, X.; Yin, M.; Sun, X.; Zhang, Z.; Yao, X.; Liu, H.; Xia, H. Hemin attenuated lps-induced acute lung injury in mice via protecting pulmonary epithelial barrier and regulating ho-1/nlrp3-mediated pyroptosis. Shock 2023, 59, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Guo, N.; Yao, W.; Jin, Y.; Gao, W.; Cai, J.; Hei, Z. Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. J. Transl. Med. 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.M.; Kamel, M.Y.; Rifaai, R.A. Effects of hemin, a heme oxygenase-1 inducer in L-arginine-induced acute pancreatitis and associated lung injury in adult male albino rats. Endocr. Regul. 2017, 51, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyu, H.; Si, H.; Li, S.; Wang, W.; Guo, J.; Li, Y.; Cao, Y.; Fu, Y.; Zhang, N. Induction of heme oxygenas-1 attenuates NLRP3 inflammasome activation in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2017, 52, 185–190. [Google Scholar] [CrossRef]
- Sun, B.; Li, G.; Guo, L.; Yin, N.; Huang, H.; Wu, X.; Huang, R.; Feng, M. Once-monthly hemin suppresses inflammatory and autoreactive CD4+ T cell responses to robustly ameliorate experimental rheumatoid arthritis. iScience 2021, 24, 103101. [Google Scholar] [CrossRef]
- Rofaeil, R.R.; Welson, N.N.; Fawzy, M.A.; Ahmed, A.F.; Atta, M.; Bahaa El-deen, M.A.; Abdelzaher, W.Y. The IL-6/HO-1/STAT3 signaling pathway is implicated in the amelioration of acetaminophen-induced hepatic toxicity: A neonatal rat model. Hum. Exp. Toxicol. 2023, 42, 9603271231151376. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T.; Omran, G.A.E.-H. Induction of heme oxygenase-1 attenuates chemotherapy-induced pulmonary toxicity in rats: A possible link between heme oxygenase-1 and NF-κB. Pak. J. Pharm. Sci. 2016, 29, 685–694. [Google Scholar] [PubMed]
- Ni, L.; Wang, Z.; Yang, G.; Li, T.; Liu, X.; Liu, C. Heme oxygenase-1 alleviates cigarette smoke-induced restenosis after vascular angioplasty by attenuating inflammation in rat model. Toxicol. Lett. 2016, 245, 99–105. [Google Scholar] [CrossRef]
- Guo, S.; Yu, M.; Fang, Q.; Zhang, L.; You, C.; Wang, X.; Liu, Y.; Han, C. Heme oxygenase-1 induction mitigates burn-associated early acute kidney injury via the TLR4 signaling pathway. Burns 2022, 48, 156–167. [Google Scholar] [CrossRef]
- Yu, X.; Han, W.; Wang, C.; Sui, D.; Bian, J.; Bo, L.; Deng, X. Upregulation of Heme Oxygenase-1 by Hemin Alleviates Sepsis-Induced Muscle Wasting in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 8927104. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, A.A.; Prawez, S.; Shakoor, A.; Ahmad, W.; Khan, A.M.; Kumar, D. Topical application of ‘Hemin’ promotes wound healing in Streptozotocin-induced diabetic rats. Vet. Arh. 2021, 91, 287–296. [Google Scholar] [CrossRef]
- Kumar, D.; Jena, G.T.; Ram, M.; Lingaraju, M.; Cholenahalli Singh, V.; Prasad, R.; Kumawat, S.; Kant, V.; Gupta, P.; Tandan, S.K.; et al. Hemin attenuated oxidative stress and inflammation to improve wound healing in diabetic rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Wang, G.-G.; Li, W.; Jiang, Y.-X.; Lu, X.-H.; Zhou, P.-P. Heme Oxygenase-1 Promotes Delayed Wound Healing in Diabetic Rats. J. Diabetes Res. 2016, 2016, 9726503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Zhong, W.; Di, C.; Lin, X.; Xia, Z. Heme Oxygenase-1 Ameliorates Dextran Sulfate Sodium-induced Acute Murine Colitis by Regulating Th17/Treg Cell Balance. J. Biol. Chem. 2014, 289, 26847–26858. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.; Meng, X.; Zhao, M.; Kang, K.; Tan, G.; Pan, S.; Luo, Y.; Liu, W.; Nan, C.; Jiang, H.; et al. Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Transl. Res. 2012, 159, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bortscher, S.; Chang, J.; Vilz, T.O.; Schäfer, N.; Sommer, N.; Wehner, S.; Kalff, J.C.; Overhaus, M. Hemin induction of HO-1 protects against LPS-induced septic ileus. J. Surg. Res. 2012, 178, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Devesa, I.; Ferrándiz, M.L.; Busserolles, J.; Alcaraz, M.J. Effects of heme oxygenase-1 inducers on established rat adjuvant arthritis. Cell. Mol. Biol. 2005, 51, 479–485. [Google Scholar] [PubMed]
- Zhang, J.; Jiang, Y.; Li, H.; Wang, J.; Li, C.; Zhang, D. Elevation of HO-1 expression protects the intestinal mucosal barrier in severe acute pancreatitis via inhibition of the MLCK/p-MLC signaling pathway. Exp. Cell Res. 2023, 424, 113508. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wu, J.; Di, C.; Xia, Z. Heme Oxygenase-1 Exerts a Protective Role in Ovalbumin-induced Neutrophilic Airway Inflammation by Inhibiting Th17 Cell-mediated Immune Response. J. Biol. Chem. 2013, 288, 34612–34626. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Balwani, M.; Anderson, K.E. Inherited Porphyrias. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–32. [Google Scholar]
- Connick, J.P.; Reed, J.R.; Cawley, G.F.; Backes, W.L. Heme oxygenase-1 affects cytochrome P450 function through the formation of heteromeric complexes: Interactions between CYP1A2 and heme oxygenase-1. J. Biol. Chem. 2021, 296, 100030. [Google Scholar] [CrossRef] [PubMed]
- Basetty, V.; Deruiter, J.; Pathak, S.; Dua, K.; Dhanasekaran, M. Advanced drug delivery systems targeting to improve therapeutic outcomes in porphyria. In Drug Delivery Systems for Metabolic Disorders; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–76. [Google Scholar]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Pritchett-Corning, K.R.; Hashway, S.; Suckow, M.A. The Laboratory Mouse, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 157–208. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Silva, I.; Solas, J.; Pinto, R.; Mateus, V. Chronic Experimental Model of TNBS-Induced Colitis to Study Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 4739. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis. JAMA 2016, 315, 762. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Voelkl, B.; Vogt, L.; Sena, E.S.; Würbel, H. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 2018, 16, e2003693. [Google Scholar] [CrossRef]
- Silva, I.; Alípio, C.; Pinto, R.; Mateus, V. Potential anti-inflammatory effect of erythropoietin in non-clinical studies in vivo: A systematic review. Biomed. Pharmacother. 2021, 139, 111558. [Google Scholar] [CrossRef]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Waterson, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwala, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; An, P.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Dillmann, W.H. The rat as a model for cardiovascular disease. Drug Discov. Today Dis. Model. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- Dietrich, M.R.; Ankeny, R.A.; Crowe, N.; Green, S.; Leonelli, S. How to choose your research organism. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2020, 80, 101227. [Google Scholar] [CrossRef]
- How to Choose the Right Mouse Models and Genetic Modification Method for Your Research. Available online: https://apac.cyagen.com/community/technical-bulletin/c57bl6-and-balbc-mouse.htm (accessed on 19 December 2023).
- C57BL/6 Mice. Available online: https://www.criver.com/products-services/find-model/c57bl6-mouse?region=3616 (accessed on 19 December 2023).
- Garg, R.; Heinzle, E.; Noor, F. Hepatocytes of Wistar and Sprague Dawley rats differ significantly in their central metabolism. J. Cell. Biochem. 2018, 119, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Pinto, S.C.; de Oliveira, R.S. Animais de Laboratório: Criação e Experimentação; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2006. [Google Scholar] [CrossRef]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef]
- Adams, D.B.; Cowan, C.W.; Marshall, M.E.; Stark, J. Competitive and territorial fighting: Two types of offense in the rat. Physiol. Behav. 1994, 55, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P.; et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; ten Boekel, E.; Rolink, A.G.; Melchers, F. B-cell development: A comparison between mouse and man. Immunol. Today. 1998, 19, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Holladay, S.D.; Smialowicz, R.J. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000, 108, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kincade, P.W. Formation of B Lymphocytes in Fetal and Adult Life. Adv. Imm. 1981, 31, 177–245. [Google Scholar] [CrossRef]
- Liu, F.; McCullough, L.D. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem. Int. 2012, 61, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R.; Hirano, S.; Cutro, B.T.; Birjandi, S.; Baila, H.; Nomellini, V.; Kovacs, E.J. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit. Care Med. 2007, 35, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Wang, C.; Han, Z.; Wang, X.; Ding, M.; Wang, Q. Experimental Rodent Models of Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 588075. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, A.; Fattahi, N.; Ramazani, A. Biomarkers: Promising and valuable tools towards diagnosis, prognosis and treatment of COVID-19 and other diseases. Heliyon 2023, 9, e13323. [Google Scholar] [CrossRef]
- Sauer, J.-M.; Porter, A.C. Preclinical biomarker qualification. Exp. Biol. Med. 2018, 243, 222–227. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age- Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.M.K.; Cheng, T.; Lee, K.T.-W.; Dissabandara, L.; Lam, A.K.-Y.; Gopalan, V. Hemin, a major heme molecule, induced cellular and genetic alterations in normal colonic and colon cancer cells. Pathol. Res. Pract. 2021, 224, 153530. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.E.; Bishop, G.M.; Robinson, S.R. Uptake and Toxicity of Hemin and Iron in Cultured Mouse Astrocytes. Neurochem Res. 2016, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]


| Hemin-Related Parameters | Animal-Related Parameters | Biomarkers Assessed | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Frequency | Route of Administration | Duration | Disease Animal Model | Specie | Strain | Gender | Age | ||
| 20 µmol/kg | 3× week | SC | 20 weeks | Emphysema | Rat | Wistar | Male | 6 weeks | IL-8, IL-10, IL-17, Macrophages, MCP-1, MDA, MIP-2α, neutrophils, SOD, GSH, TNF-α | [18] |
| 30 µmol/kg | IP | 8 weeks | Liver fibrosis | Mice | C57BL/6 | ND | HO-1, NFAT5, ROS, TGF-β1 | [19] | ||
| Steatohepatitis and fibrosis | 8 weeks | HO-1, MCP-1, NF-kB, TGF-β1 | [20] | |||||||
| Single dose | - | LPS-stimulated inflammatory response | Rat | Sprague Dawley | 10–11 weeks | HO-1, IL-1β, IL-6, LDH, MDA, SOD | [21] | |||
| Wistar | Female | [22] | ||||||||
| 40 µmol/kg | Daily | 8 days | Nephrotoxicity | Albino | Male | Adult | GSH, HO-1, IL-6, MDA | [23] | ||
| Single dose | - | Acute liver failure | Sprague Dawley | 6–7 weeks | HO-1, IL-1β, IL-6, MDA, MPO, NF-kB p65, Nrf2, TNF-α | [24] | ||||
| SC | Reproductive toxicity | Wistar | Adult | CAT, HO-1, GSH, iNOS, MDA, NO, TNF-α | [25] | |||||
| ND | 30 days | Diabetes | ND | CAT, GSH, HO-1, MDA, MPO, SOD, TNF-α | [26] | |||||
| 75 µmol/kg | Daily | IP | 2 days | Neutrophilic airway inflammation | Mice | BALB/c and DO11.10 transgenic | Female | 8–10 weeks | HO-1, IL-6, IL-10, IL-17A, JAK1, JAK2, STAT3, T-cells | [27] |
| Variable (days −2, −1, 12, 13, 21, 22, and 25) | 27 days | Eosinophilic asthma | BALB/c | ND | ND | GATA3, HO-1, IL-4, IL-5, IL-17A, IL-17F, eosinophils, lymphocytes, macrophages, neutrophils, RORγt, SOCS3, STAT3 | [28] | |||
| 27 days | Allergic airway inflammation | BALB/c, DO11.10, and Bas-TRECK transgenic | 6–8 weeks | Basophils, eosinophils, IL-4, T-cells | [29] | |||||
| 80 µmol/kg | Single dose | - | Acute pulmonary inflammation | CD1 (WT and A2A gene-deficient) and C57BL/6 (WT and A2B gene-deficient) | Male | 8–12 weeks | HO-1, IL-6, TNF-α | [30] | ||
| 8 × 104 µmol/kg | Acute lung injury | C57BL/6 (WT and HO-1flox/flox) | HO-1, IL-6, NF-kB p65, NF-kB p52, TNF-α | [31] | ||||||
| 7.5 × 10−2 mg/kg | Acute pancreatitis | Rat | Sprague Dawley | ND | HO-1, IL-10, TNF-α | [32] | ||||
| 6–7 weeks | [33] | |||||||||
| 0.1, 0.3, or 1.0 mg/kg | PI | Pleurisy | Wistar | ND | CAT, GPx, GST, IL-1β, iNOS, MCP-1, MPO, NOx, SOD, TNF-α | [34] | ||||
| 0.1, 0.3, 1, or 3 mg/kg | Daily | IV | 3 days | Influenza pneumonia | Mice | BALB/c | Female | 5–6 weeks | IFN-γ, IL-6, IL-10, HO-1, MCP-1, TNF-α | [35] |
| 1, 3, or 10 mg/kg | Single dose | SC | - | Temporomandibular joint arthritis | Rat | Wistar | Male | ND | HO-1, IL-1β, MPO, TNF-α, White blood cells | [36] |
| 5 mg/kg | Daily | IG | 9 days | Chronic gastric ulcers | 8–9 weeks | COX-2, HO-1, HO-2, IL-1β, iNOS, Nrf2, TNF-α | [37] | |||
| Single dose | IP | - | Peritonitis | Mice | C57BL/6 | 6–8 weeks | IL-6, Leukocytes, macrophages, polymorphonuclear cells, TNF-α | [38] | ||
| Renal ischemia–reperfusion injury | ND | 8–12 weeks | HO-1, IL-1β, IL-6, Neutrophils, nitrotyrosine, peroxidase, TNF-α | [39] | ||||||
| 5 or 10 mg/kg | Daily | 14 days | Experimental colitis | CD-1 | Female | 6 weeks | IL-10, TNF-α | [8] | ||
| 4 days | Male | 6–10 weeks | IL-1β, IL-10, MPO, TNF-α | [7] | ||||||
| 8 mg/kg | Single dose | - | Sepsis | Rat | Sprague Dawley | 8–10 weeks | Caspase-1, Caspase-11, GSDMD, HMGB1, IL-1β, IL-18, TNF-α | [40] | ||
| 10 mg/kg | Sepsis | Mice | C57BL/6 | ND | GDH, HO-1, IL-1β, IL-6, LDH, MDA, SOD2, TLR4 | [41] | ||||
| ND | Renal inflammation and vasoconstrictor dysfunction | Rat | Wistar | 10–13 weeks | HO-1, IL-1β, iNOS, NF-kB | [42] | ||||
| 10 or 30 mg/kg | Daily | IP | 14 days | Neurotoxicity | ND | CAT, GSH, HO-1, IL-1β, TNF-α | [43] | |||
| 20 mg/kg | Every 2 days | 7 days | Experimental colitis | Mice | C57BL/6 | Female | 6–8 weeks | 4-HNE, IFN-γ, IL-1β, IL-6, IL-12, iNOS, Ly6C, macrophages, MDA, MPO, NO, NF-kB p-p65, STAT1, STAT6, TNF-α | [44] | |
| 14 days | Renal failure | Rat | Wistar | Male | ND | 4-HNE, GSH, GSH-Px, HO-1, MPO, SOD | [45] | |||
| 25 mg/kg | Daily | 5 days | Myocardial infarction | ND | eNOS, GSH, HO-1, LDH, MDA, TNF-α | [46] | ||||
| 3× week | ND | Endometrial hyperplasia | Female | 9–10 weeks | GSH, IL-1β, iNOS, MDA, NOx, SOD | [47] | ||||
| Every 2 days | 6 days | Vascular dysfunction | Mice | C57BL/6 | Male | 6–12 weeks | 3-nitrotyrosine, 4-HNE, eNOS, Hmox1, macrophages, HO-1, IL-6, ROS | [48] | ||
| 30 mg/kg | 30 days | Penetrating keratoplasty | Rat | Wistar | Female | 6–8 weeks | HO-1, iNOS, macrophages, MCP-1, MIP-1α, TNF-α | [49] | ||
| 2× week | 8 weeks | Obesity | Zucker fatty and Zucker lean | Male | 12 weeks | 8-isoprostane, HO-1, IL-1β, IL-6, macrophages, nitrotyrosine, TGF-β1/2/3, TNF-α | [50] | |||
| Single dose | - | Acute lung injury | Mice | BALB/c | Female | ND | Caspase-1, IL-1β, MDA, MPO, HO-1, ROS | [51] | ||
| Rat | Sprague Dawley | Male | HO-1, IL-6, MDA, MPO, NF-kB, Nrf2, SOD, TNF-α | [52] | ||||||
| Acute pancreatitis | 16 weeks | HO-1, MDA, NO, TNF-α | [53] | |||||||
| Liver transplantation | Wistar | ND | HO-1, IL-6, MDA, NF-kB, Nrf2, SOD, TNF-α | [54] | ||||||
| ND | Mastitis | Mice | BALB/c | Female | 8–12 weeks | IL-1β, MPO, NLRP3, procaspase-1, ROS, TXNIP | [55] | |||
| 40 mg/kg | 2× week | IP | 14 days | Rheumatoid arthritis | Rat | Sprague Dawley | Male | 8–10 weeks | IFN-γ, IL-1β, IL-6, T-cells, TNF-α | [56] |
| 40 or 90 mg/kg | 28 days | |||||||||
| 40 mg/kg + 120 mg/kg | 2 daily adm + 1 adm | IP + SC | 28 or 41 days | |||||||
| 50 mg/kg | Daily | IP | 14 days | Hepatic toxicity | N.D. | 1 week | GSH, HO-1, IL-1β, IL-6, MDA, NOx, STAT3, TNF-α | [57] | ||
| 14 days | Pulmonary toxicity | Wistar | Female | Adult | GSH, HO-1, IL-6, LDH, MDA, MPO, NF-kB, NO, SOD | [58] | ||||
| Every 2 days | 21 days | Restenosis | Sprague Dawley | Male | ND | CD45, HO-1, IL-1β, IL-6, MCP-1, NF-kB, TNF-α | [59] | |||
| Single dose | - | Major burn model | Adult | HO-1, IL-1β, IL-6, MDA, NF-kB p65, TLR4, TNF-α | [60] | |||||
| Acute kidney injury | Sprague Dawley (WT and PINK1 knockout) | 8 weeks | Caspase-1, HO-1, IL-1β, IL-6, MDA, NGAL, SOD, TNF-α | [61] | ||||||
| Sepsis | Mice | C57BL/6 | HO-1, IL-6, MDA, SOD, TNF-α | |||||||
| 0.5% | Daily | Topical | 13 days | Diabetes | Rat | Wistar | Adult | IL-10, TNF-α | [62] | |
| 2× day | 19 days | CAT, GPx, GSH, HO-1, IL-10, MDA, SOD, TNF-α | [63] | |||||||
| 10% | N.D. | 21 days | Sprague Dawley | ND | HO-1, IL-6, MDA, SOD, TNF-α | [64] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estarreja, J.; Caldeira, G.; Silva, I.; Mendes, P.; Mateus, V. The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: A Systematic Review. Biomedicines 2024, 12, 898. https://doi.org/10.3390/biomedicines12040898
Estarreja J, Caldeira G, Silva I, Mendes P, Mateus V. The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: A Systematic Review. Biomedicines. 2024; 12(4):898. https://doi.org/10.3390/biomedicines12040898
Chicago/Turabian StyleEstarreja, João, Gonçalo Caldeira, Inês Silva, Priscila Mendes, and Vanessa Mateus. 2024. "The Pharmacological Effect of Hemin in Inflammatory-Related Diseases: A Systematic Review" Biomedicines 12, no. 4: 898. https://doi.org/10.3390/biomedicines12040898