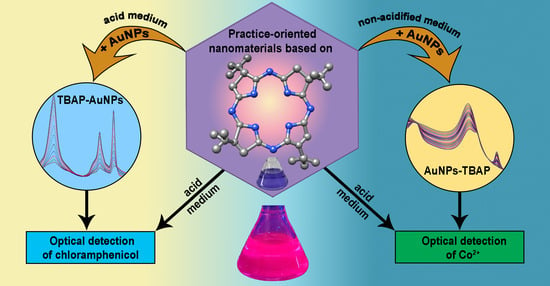
Nanomaterials Based on 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine Exhibiting Bifunctional Sensitivity for Monitoring Chloramphenicol and Co2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
3. Results and Discussion
3.1. UV–Vis Study Regarding 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine (TBAP)
3.2. Fluorescence Study of 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine (TBAP)
3.3. 1H-NMR Study of the TBAP Azaporphyrin
3.4. The Optical Detection of CHL by UV–Vis Spectroscopy Used as a Sensitive Substance, TBAP, in Acid Medium
3.5. The Detection of CHL Using a Plasmonic Complex Formed between Acidified TBAP and AuNPs
3.5.1. Obtaining and Characterization of AuNPs
3.5.2. Method for Obtaining the Complex between TBAP and AuNPs
3.5.3. The Optical Detection of CHL Using the Hybrid Material AuNPs–TBAP
3.5.4. Presumptions for the Mechanism of Detection of CHL Using a Plasmonic Complex Formed between Acidified TBAP and AuNPs
3.5.5. Effect of Potentially Interfering Species in the CHL Detection
3.6. Detection of Co(II) from Aqueous Media Using the AuNPs–TBAP Complex, Generated in Water–DMSO Solution
3.6.1. AuNPs–TBAP Complex Formation in Water–DMSO
3.6.2. UV–Vis Detection of Co2+ Using AuNPs–TBAP Complex
3.6.3. Potential Mechanism of Co2+ Detection Based on Compared FT-IR Spectra of AuNPs–TBAP Complex and AuNPs–TBAP-Co2+
3.6.4. Effect of Interfering Species for the UV–Vis Detection of Co2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, M.; Paolini, E.; Meroni, M.; Dongiovanni, P. Cutting-Edge Therapies and Novel Strategies for Acute Intermittent Porphyria: Step-by-Step towards the Solution. Biomedicines 2022, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Shinmori, H.; Kawabata, S.; Sugikawa, K.; Funabashi, H.; Kuroda, A.; Ikeda, A. High photodynamic activities of water-soluble inclusion complexes of 5,15-diazaporphyrins in cyclodextrin. Org. Biomol. Chem. 2019, 17, 3141–3149. [Google Scholar] [CrossRef]
- Kaushik, A.; Bhandary, S.; Sharma, G.D.; Sankar, J. Aza-benzannulated-perylenebisimide-porphyrin dyad as an intensely absorbing donor in bulk-heterojunction organic solar cells. Mater. Adv. 2023, 4, 6358–6366. [Google Scholar] [CrossRef]
- Koczorowski, T.; Cerbin-Koczorowska, M.; Rębiś, T. Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing—A Review. Nanomaterials 2021, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Zeyada, H.M.; El-Nahass, M.M.; Makhlouf, M.M. Electronic transport mechanisms in tetraphenyleprophyrin thin films. Curr. Appl. Phys. 2011, 11, 1326–1331. [Google Scholar] [CrossRef]
- Al-Zubaidi, A.A.; Elfaki, A.A.A.; Darwish, A.A.A. Influence of film thickness on structural and optical properties of 2,7,12,17-tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine nanostructure thin films for optical applications. J. Mol. Struct. 2020, 1218, 128499. [Google Scholar] [CrossRef]
- Alsharari, A.M.; Darwish, A.A.A.; Rashad, M. Formation of flexible nano-organic films of 2,7,12,17-tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine for promising optoelectronic applications. Opt. Mater. 2020, 105, 109870. [Google Scholar] [CrossRef]
- Lorenzo, D. Chloramphenicol Resurrected: A Journey from Antibiotic Resistance in Eye Infections to Biofilm and Ocular Microbiota. Microorganisms 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, P.; Missiri, D.; Kournoutou, G.; Sazakli, E.; Papadopoulos, G.; Papaioannou, D.; Dinos, G.P.; Athanassopoulos, C.M.; Kalpaxis, D.L. New Chloramphenicol Derivatives from the Viewpoint of Anticancer and Antimicrobial Activity. Antibiotics 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N. Recent Trends in Synthesis of Chloramphenicol New Derivatives. Antibiotics 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Sathya, A.; Prabhu, T.; Ramalingam, S. Structural, biological and pharmaceutical importance of antibiotic agent chloramphenicol. Heliyon 2020, 6, e03433. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.M.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: A review. Environ. Chem. Lett. 2022, 20, 1929–1963. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, F.; Duan, H.; Dong, F.; Chen, X.; Feng, M.; Zhang, Z.; Cizmas, L.; Sharma, V.K. Degradation of chloramphenicol by chlorine and chlorine dioxide in a pilot-scale water distribution system. Sep. Purif. Technol. 2018, 211, 564–570. [Google Scholar] [CrossRef]
- Abdollahi, M.; Mostafalou, S. Chloramphenicol. In Encyclopedia of Toxicology; Elsevier, Inc.: Philadelphia, PA, USA, 2014; pp. 837–840. [Google Scholar] [CrossRef]
- Dowling, P.M. Chloramphenicol, Thiamphenicol, and Florfenicol. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Inc.: New York, NY, USA, 2013; pp. 269–277. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Rockenbauer, M.; Sørensen, H.T.; Olsen, J. A population-based case ± control teratologic study of oral chloramphenicol treatment during pregnancy. Eur. J. Epidemiol. 2000, 16, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhou, H.; Liu, Q. PCN-222 MOF decorated conductive PEDOT films for sensitive electrochemical determination of chloramphenicol. Mater. Chem. Phys. 2021, 270, 124831. [Google Scholar] [CrossRef]
- Li, H.K.; Ye, H.L.; Zhao, X.X.; Sun, X.L.; Zhu, Q.Q.; Han, Z.Y.; Yuan, R.; He, H. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere 2021, 275, 130096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Deka, A.K.; Kumar, K.J.; Basumatary, S. Monitoring Strategies for Heavy Metals in Foods and Beverages: Limitations for Human Health Risks. In Heavy Metals—Recent Advances; Almayyahi, B.A., Ed.; InTech Open: Rijeka, Croatia, 2023; Volume 35. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, E.K.; Prodromidis, M.I.; Giokas, D.L.; Pappas, A.C.; Karayannis, M.I. Highly selective spectrophotometric determination of trace cobalt and development of a reagentless fiber-optic sensor. Anal. Chim. Acta 2002, 467, 205–215. [Google Scholar] [CrossRef]
- Jiang, Z.-T.; Li, R.; Zhang, J.-C. Determination of cobalt in foods using β-cyclodextrin epichlorohydrin polymer functionalized with 1-(2-pyridylazo)-2-naphthol. JFDA 2004, 12, 7. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, Y.; Hu, Y.; Chen, Y.; Xia, L.; Li, G. Liquid-Phase Cyclic Chemiluminescence for the Identification of Cobalt Speciation. Anal. Chem. 2024, 96, 3933–3941. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Genchi, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Saulea, M.; Stoica, A.I.; Baiulescu, G.E.; Marinescu, D.; Ionica, M. Determination of cobalt in food samples. Rev. Chim. 2004, 55, 301–303. [Google Scholar]
- López, O.N.B.; Fuentes, H.C.; Perezgasga, F.V.; Casillas, H.A.M.; Chamarro, J.A. Detection and analysis of cobalt in continuous flow using an analytical microsystem based on LTCC technology. Sens. Actuators B Chem. 2016, 227, 11–16. [Google Scholar] [CrossRef]
- Zaidan, K.H.; Al-Terehi, M.; AL-Mamoori, M.J.A.; Al-Shuhaib, S.M.B.; Al-Saadi, A.H.; Gathwan, H.K. Detection some trace elements in human milk and effect of some factors on its concentrations. JBMS 2013, 1, 6–12. [Google Scholar]
- Vashisht, D.; Kaur, K.; Jukaria, R.; Vashisht, A.; Sharma, S.; Mehta, S.K. Colorimetric chemosensor based on Coumarin skeleton for selective naked eye detection of Cobalt (II) ion in near aqueous medium. Sens. Actuators B Chem. 2019, 280, 219–226. [Google Scholar] [CrossRef]
- Li, C.L.; Huang, C.C.; Periasamy, A.P.; Roy, P.; Wu, W.C.; Hsu, C.L.; Chang, H.T. Synthesis of photoluminescent carbon dots for the detection of cobalt ions. RSC Adv. 2015, 5, 2285–2291. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Lanzante, J.R. A Cautionary Note on the Use of Error Bars. J. Clim. 2005, 18, 3699–3703. [Google Scholar] [CrossRef]
- Al-Zubaidi, A.A.; Elfaki, A.A.A.; Al-Ghamdi, S.A.; Darwish, A.A.A. Structural analytical and electrical conduction mechanisms of 2,7,12,17-tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine nanostructure films. J. Mater. Sci. Mater. Electron. 2021, 32, 10070–10077. [Google Scholar] [CrossRef]
- Ovchenkova, E.N.; Hanack, M.; Lomova, T.N. Synthesis and Characterization of Some Five-Coordinated Tetraazaporphyrin and Phthalocyanine Manganese(III) Complexes. Macroheterocycles 2010, 3, 63–67. [Google Scholar] [CrossRef]
- Sawamura, T.; Tanaka, T.; Ishige, H.; Iizuka, M.; Murayama, Y.; Otsuji, E.; Ohkubo, A.; Ogura, S.-I.; Yuasa, H. The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency. Int. J. Mol. Sci. 2015, 16, 22415–22424. [Google Scholar] [CrossRef] [PubMed]
- Mone, M.; Tang, S.; Genene, Z.; Murto, P.; Jevric, M.; Zou, X.; Ràfols-Ribé, J.; Abdulahi, B.A.; Wang, J.; Mammo, W.; et al. Near-Infrared Emission by Tuned Aggregation of a Porphyrin Compound in a Host–Guest Light-Emitting Electrochemical Cell. Adv. Opt. Mater. 2021, 9, 2001701. [Google Scholar] [CrossRef]
- Furuyama, T.; Satoh, K.; Kushiya, T.; Kobayashi, N. Design, Synthesis, and Properties of Phthalocyanine Complexes with Main-Group Elements Showing Main Absorption and Fluorescence beyond 1000 nm. J. Am. Chem. Soc. 2013, 136, 765–776. [Google Scholar] [CrossRef] [PubMed]
- McKearney, D.; Zhou, W.; Zellman, C.; Williams, V.E.; Leznoff, D.B. Facile tuning of strong near-IR absorption wavelengths in manganese(iii) phthalocyanines via axial ligand exchange. Chem. Comm. 2019, 55, 6696–6699. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Murata, K.; Ishii, K. Distorted Porphyrins with High Stability: Synthesis and Characteristic Electronic Properties of Mono- and Di-Nuclear Tricarbonyl Rhenium Tetraazaporphyrin Complexes. Chem.-Eur. J. 2021, 27, 8994–9002. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, G.Q.; Ji, S.; Zhang, Y.; Li, J.; Ou, H.; Gao, Z.; Feng, G.; Ding, D. Activatable Persistent Luminescence from Porphyrin Derivatives and Supramolecular Probes with Imaging-Modality Transformable Characteristics for Improved Biological Applications. Angew. Chem. Int. Ed. 2022, 61, e202116174. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D.; Raines, R.T. Bright Ideas for Chemical Biology. ACS Chem. Bio 2008, 3, 142. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N. Meso-Azaporphyrins and Their Analogues. In The Porphyrin Handbook; Kadish, K., Smith, K.M., Guilard, R., Eds.; Elsevier: London, UK, 1999; Volume 2, pp. 314–333. ISBN 0123932025. [Google Scholar]
- Arpita, R.; Magdaong, N.C.M.; Jing, H.; Rong, J.; Diers, J.R.; Kang, H.S.; Niedzwiedzki, D.M.; Taniguchi, M.; Kirmaier, C.; Lindsey, J.S.; et al. Balancing Panchromatic Absorption and Multistep Charge Separation in a Compact Molecular Architecture. J. Phys. Chem. A 2022, 126, 9353–9365. [Google Scholar] [CrossRef]
- Pham, H.T.; Yoo, J.; VandenBerg, M.; Muyskens, M.A. Fluorescence of Scopoletin Including its Photoacidity and Large Stokes Shift. J. Fluoresc. 2019, 30, 71–80. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Mirica, M.C.; Balcu, I.; Bucovicean, C.; Cretu, C.; Armeanu, I.; Fagadar-Cosma, G. Syntheses, Spectroscopic and AFM Characterization of Some Manganese Porphyrins and Their Hybrid Silica Nanomaterials. Molecules 2009, 14, 1370–1388. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Castellano, F.N.; Gryczynski, I.; Gryczynski, Z.; Dattelbaum, J.D. Two-photon excitation of rhenium metal–ligand complexes. J. Photochem. Photobiol. A Chem. 1999, 122, 95–101. [Google Scholar] [CrossRef]
- Zannotti, M.; Giovannetti, R.; Minofar, B.; Řeha, D.; Plačková, L.; D’Amato, C.A.; Rommozzi, E.; Dudko, H.V.; Kari, N.; Minicucci, M. Aggregation and metal-complexation behaviour of THPP porphyrin in ethanol/water solutions as function of pH. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawis, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Patnaik, A. Non-covalent approaches to facile synthesis of dimension-specific H and J-aggregates. In J-Aggregates; Kobayashi, T., Ed.; World Scientific Publishing: Singapore, 2012; Volume 2, pp. 343–402. [Google Scholar]
- Dehghani Sanij, M.; Bahrampour, A.; Bahrampour, A.R. Resonant Light Scattering toward Optical Fiber Humidity Sensors. Photonic Sens. 2018, 9, 60–68. [Google Scholar] [CrossRef]
- Bhopate, D.P.; Kim, K.; Mahajan, P.G.; Gore, A.H.; Patil, S.R.; Majhi, S.M.; Naik, G.K.; Liang, T.-T.; Ahemad, J.-M.; Yu, Y.-T.; et al. Fluorescent chemosensor for quantitation of multiple atmospheric gases. J. Nanomed. Nanotechnol. 2017, 8, 1000436. [Google Scholar] [CrossRef]
- El Bakouri, O.; Szczepanik, D.W.; Jorner, K.; Ayub, R.; Bultinck, P.; Solà, M.; Ottosson, H. Three-Dimensional Fully π-Conjugated Macrocycles: When 3D-Aromatic and When 2D-Aromatic-in-3D? J. Am. Chem. Soc. 2022, 144, 8560–8575. [Google Scholar] [CrossRef] [PubMed]
- Matamala-Cea, E.; Valenzuela-Godoy, F.; González, D.; Arancibia, R.; Dorcet, V.; Hamon, J.R.; Novoa, N. Efficient preparation of 5, 10, 15, 20-tetrakis (4-bromophenyl) porphyrin. Microwave assisted v/s conventional synthetic method, X-ray and hirshfeld surface structural analysis. J. Mol. Struct. 2020, 1201, 127139. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; Yu, Z.G.; Wang, K.; Hu, B. Magnetic field effects on excited states, charge transport, and electrical polarization in organic semiconductors in spin and orbital regimes. Adv. Phys. 2019, 68, 49–121. [Google Scholar] [CrossRef]
- Cartigny, B.; Azaroual, N.; Imbenotte, M.; Sadeg, N.; Testart, F.; Richecoeur, J.; Vermeersch, G.; Lhermitte, M. 1H NMR Spectroscopic Investigation of Serum and Urine in a Case of Acute Tetrahydrofuran Poisoning. J. Anal. Toxicol. 2001, 25, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Li, Z.; Dong, C.; Li, H.W.; Li, J. Electrochemical detection of chloramphenicol using palladium nanoparticles decorated reduced graphene oxide. Microchem. J. 2019, 148, 774–783. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Sebarchievici, I.; Lascu, A.; Creanga, I.; Palade, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, G. Optical and electrochemical behavior of new nano-sized complexes based on gold-colloid and Co-porphyrin derivative in the presence of H2O2. J. Alloys Compd. 2016, 686, 896–904. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; DePenning, R.; Turner, M.; Padalkar, S. Effect of citrate ratio and temperature on gold nanoparticle size and morphology. Mater. Res. Express 2016, 3, 105027. [Google Scholar] [CrossRef]
- Epuran, C.; Fratilescu, I.; Macsim, A.M.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors 2022, 10, 133. [Google Scholar] [CrossRef]
- Fringu, I.; Lascu, A.; Macsim, A.-M.; Fratilescu, I.; Epuran, C.; Birdeanu, M.; Fagadar-Cosma, E. Pt(II)-A2B2 metalloporphyrin-AuNPS hybrid material suitable for optical detection of 1-anthraquinonsulfonic acid. Chem. Pap. 2022, 76, 2513–2527. [Google Scholar] [CrossRef]
- Lascu, A.; Vlascici, D.; Birdeanu, M.; Epuran, C.; Fratilescu, I.; Fagadar-Cosma, E. The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine. Int. J. Mol. Sci. 2023, 24, 17265. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wu, C.; Zhou, Z.; Fu, L.; Bai, Y. Fluorescent Sensing of Ciprofloxacin and Chloramphenicol in Milk Samples via Inner Filter Effect and Photoinduced Electron Transfer Based on Nanosized Rod-Shaped Eu-MOF. Foods 2022, 11, 3138. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Du, X.; Ding, Y.; Li, H. Establishment of a dual-aptasensor for simultaneous detection of chloramphenicol and kanamycin. Food Addit. Contam. Part A 2021, 38, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Dhayanithi, C.A.; Palpandi, K.; Raman, N.; Babu, S.G. Development of amine-based transition metal MOFs as efficient electrochemical sensors for the detection of chloramphenicol in food and pharmaceutical samples. Electrochim. Acta 2023, 470, 143358. [Google Scholar] [CrossRef]
- Hassan, M.M.; He, P.; Xu, Y.; Zareef, M.; Li, H.; Chen, Q. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration. Food Chem. 2022, 374, 131765. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.A.; Islam, R.; Shoeb, M.; Nahar, N. Determination of chloramphenicol in meat samples using liquid chromatography–tandem mass spectrometry. Food Sci. Nutr. 2021, 9, 5670–5675. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Liu, C.Q.; Li, L.; Xiang, M. The occurrence of chloramphenicol and tetracyclines in municipal sewage and the Nanming River, Guiyang City, China. J. Environ. Monit. 2009, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Yang, M.; Zhu, M.; Chen, W.; Li, Q.; Sun, D.; Bi, X.; Maletskyi, Z.; Ratnaweera, H. Detection Limits of Antibiotics in Wastewater by Real-Time UV–VIS Spectrometry at Different Optical Path Length. Processes 2022, 10, 2614. [Google Scholar] [CrossRef]
- Jain, P.S.; Chaudhari, A.J.; Patel, S.A.; Patel, Z.N.; Patel, D.T. Development and validation of the UV-spectrophotometric method for determination of terbinafine hydrochloride in bulk and in formulation. Pharm. Methods 2011, 2, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Baxter, A.; Young, P.; Kennedy, G.; Elliott, C.; Weigel, S.; Gatermann, R.; Ashwin, H.; Stead, S.; Sharman, M. Detection of chloramphenicol and chloramphenicol glucuronide residues in poultry muscle, honey, prawn and milk using a surface plasmon resonance biosensor and Qflex® kit chloramphenicol. Anal. Chim. Acta 2005, 529, 109–113. [Google Scholar] [CrossRef]
- Lindberg, A.A.; Nilsson, L.H.; Bucht, H.; Kallings, L.O. Concentration of chloramphenicol in the urine and blood in relation to renal function. BMJ 1966, 2, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Nakashima, S. Changes in IR band areas and band shifts during water adsorption to lecithin and ceramide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 228, 117779. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Jemmis, E.D. Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Gorski, A.; Gawinkowski, S.; Starukhin, A.; Gladkov, L.; Chizhova, N.; Mamardashvili, N.; Scheblykin, I.; Waluk, J. Resonance Raman and FTIR spectra of Mg-porphyrazines. J. Mol. Struct. 2014, 1058, 197–204. [Google Scholar] [CrossRef]
- Epuran, C.; Fratilescu, I.; Anghel, D.; Birdeanu, M.; Orha, C.; Fagadar-Cosma, E. A Comparison of Uric Acid Optical Detection Using as Sensitive Materials an Amino-Substituted Porphyrin and Its Nanomaterials with CuNPs, PtNPs and Pt@CuNPs. Processes 2021, 9, 2072. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, W.; Shi, D.; Li, X.; Zhang, H.; Chen, M. Porphyrin Derivative Conjugated with Gold Nanoparticles for Dual-Modality Photodynamic and Photothermal Therapies In Vitro. ACS Biomater. Sci. Eng. 2018, 4, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, D.; Li, M.; Yao, Q.; Tian, R.; Long, S.; Peng, X. NIR aza-pentamethine dyes as photosensitizers for photodynamic therapy. Dyes Pigment 2020, 177, 108284. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Review of porphyrin-based photodynamic therapy materials. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Swamy, C.A.P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Tian, Z.; Liu, X.; Guo, Y.; He, J.; Wang, Z.; Zhou, T.; Liu, Y. Porphyrin-Based Nanoparticles: A Promising Phototherapy Platform. ChemPlusChem 2022, 87, e202200156. [Google Scholar] [CrossRef]
- Queirós, C.; Leite, A.; Moura, N.M.M.; Cerqueira, A.F.R.; Serra, V.V.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.G. Exploring the reactivity of formylporphyrins with 3-(diethylamino)phenol. Synthesis, spectroscopic properties and singlet oxygen generation of a new porphyrin–rosamine conjugate. Dyes Pigment 2023, 217, 111431. [Google Scholar] [CrossRef]
- Kudoh, Y.; Suzuki, E.; Ochiai, H.; Ise, K.; Kimura, Y.; Minoura, M.; Nakano, H.; Matano, Y. Synthesis, Structure, and Redox and Optical Properties of 5,10,15,20-Tetraaryl-5-azaporphyrinium Salts. Chem. Eur. J. 2023, 29, e202302148. [Google Scholar] [CrossRef] [PubMed]
- Hokin, B.; Adams, M.; Ashton, J.; Louie, H. Analysis of the cobalt content in Australian foods. Asia Pac. J. Clin. Nutr. 2004, 13, 284–288. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, Y.; Jiang, J. Infrared spectra of metal-free, N′,N-dideuterio, and magnesium porphyrins: Density functional calculations. SSA 2005, 61, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bai, J.; Huo, Y.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol. Biosens Bioelectron. 2019, 149, 111801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, L.; Li, H.; Zhao, L.; Chen, T.; Liu, J.; Gao, Y.; Pan, H. An electrochemical aptasensor for the detection of chloramphenicol based on ultra-small Au-inserted hollow PCN-222 MOF. Microchim Acta 2023, 190, 366. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.P.; Zhang, Y.C.; Zhang, X.; Shen, L. Green preparation of chlorine-doped graphene and its application in electrochemical sensor for chloramphenicol detection. SN Appl. Sci. 2019, 1, 157. [Google Scholar] [CrossRef]
- Umamaheswari, R.; Manavalan, S.; Chen, S.M.; Govindasamy, M.; Chen, T.W.; Maiyalagan, T. Microwave-assisted synthesis of europium(III) oxide decorated reduced graphene oxide nanocomposite for detection of chloramphenicol in food samples. Compos B Eng. 2018, 161, 29–36. [Google Scholar] [CrossRef]
- Jayan, H.; Sun, D.W.; Pu, H.; Wei, Q. Mesoporous silica coated core-shell nanoparticles substrate for size-selective SERS detection of chloramphenicol. Spectrochim. Acta A Mol. Biomol. 2023, 284, 121817. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lai, G.; Zhang, H.; Yu, A. Amperometric aptasensing of chloramphenicol at a glassy carbon electrode modified with a nanocomposite consisting of graphene and silver nanoparticles. Microchim. Acta 2017, 184, 1445–1451. [Google Scholar] [CrossRef]
- Gao, L.L.; Li, S.P.; Wang, Y.; Wu, W.N.; Zhao, X.L.; Li, H.J.; Xu, Z.H. Quinoline-based hydrazone for the colorimetric detection of Co2+ and fluorescence turn-on response of Zn2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118025. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chang, D.; Zhang, G.; Zhang, C.; Zhang, Y.; Dong, C.; Chu, L.; Shuang, S. Co2+ detection, cell imaging, and temperature sensing based on excitation-independent green-fluorescent N-doped carbon dots. RSC Advances 2019, 9, 41361–41367. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Ahmad, M.N.; Normaya, E. A highly sensitive and selective thiosemicarbazone chemosensor for detection of Co2+ in aqueous environments using RSM and TD/DFT approaches. Sci Rep. 2021, 11, 20963. [Google Scholar] [CrossRef] [PubMed]
- Temel, N.K.; Çöpür, M. Determination of trace cobalt (II) in spices samples by ultrasonic assisted cloud point extraction with spectrophotometry. J. Mol. Struct. 2023, 1284, 135433. [Google Scholar] [CrossRef]
- Tavallali, H.; Deilamy-Rad, G.; Parhami, A.; Mousavi, S.Z. A novel development of dithizone as a dual-analyte colorimetric chemosensor: Detection and determination of cyanide and cobalt (II) ions in dimethyl sulfoxide/water media with biological applications. J. Photochem. Photobiol. B Biol. 2013, 125, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.H.; Gunjal, D.B.; Kokate, M.R.; Sudarsan, V.; Anbhule, P.V.; Patil, S.R.; Kolekar, G.B. Highly Selective and Sensitive Recognition of Cobalt(II) Ions Directly in Aqueous Solution Using Carboxyl-Functionalized CdS Quantum Dots as a Naked Eye Colorimetric Probe: Applications to Environmental Analysis. ACS Appl Mater Interfaces. 2012, 4, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, F.; Pan, Y. Chemiluminescence detection of trace amounts of cobalt (II) with the 2,6,7-trihydroxy-9-(4′-chlorophenyl)-3-fluorone–hydrogen peroxide–cetyltrimethylammonium bromide system. The Analyst 1998, 123, 273–275. [Google Scholar] [CrossRef]
- Farahani, T.Z.; Bagherian, G.; Chamjangali, M.A.; Ashrafi, M. On-line determination of the trace amount of cobalt(II) in real samples by flame atomic absorption spectrometry method after pre-concentration by modified polyvinyl chloride. SN Appl. Sci. 2023, 5, 183. [Google Scholar] [CrossRef]
- Antohe, I. Cobalt Ions Detection Using an Evanescent Wave Optical Fiber Sensor. Rom. Rep. Phys. 2022, 74, 505. [Google Scholar]
- Talio, M.C.; Alesso, M.; Acosta, M.G.; Acosta, M.; Fernández, L.P. Sequential determination of lead and cobalt in tap water and foods samples by fluorescence. Talanta 2014, 127, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.; Keskin, C.S.; Özdemir, A. Highly sensitive determination of Co(II) ions in solutions by using modified silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118487. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, H.H.; Amin, S.A.; Moustafa, E.M.I. Utilization of a plasticized PVC optical sensor for the selective and efficient detection of cobalt (II) in environmental samples. RSC Adv. 2022, 12, 18431–18440. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, C.B.; Rojas, F.S.; Pavón, J.M.C. Determination of Cobalt in Food, Environmental and Water Samples with Preconcentration by DispersiveLiquid-Liquid Microextraction. Am. J. Anal. Chem. 2012, 3, 125–130. [Google Scholar] [CrossRef]
- Wang, S.; Meng, S.; Guo, Y. Cloud Point Extraction for the Determination of Trace Amounts of Cobalt in Water and Food Samples by Flame Atomic Absorption Spectrometry. J. Spectrosc. 2013, 2013, 735702. [Google Scholar] [CrossRef]
- Tekin, Z.; Unutkan, T.; Erulaş, F.; Bakırdere, E.G.; Bakırdere, S. A green, accurate and sensitive analytical method based on vortex assisted deep eutectic solvent-liquid phase microextraction for the determination of cobalt by slotted quartz tube flame atomic absorption spectrometry. Food Chem. 2019, 310, 125825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yang, L.; Li, L.; Guo, Y.-Q.; Pang, X.X.; Li, P.; Ye, F.; Fu, Y. A New Fluorescent Chemosensor for Cobalt (II) Ions in Living Cells Based on 1,8-Naphthalimide. Molecules 2019, 24, 3093. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Jang, S.C.; Kim, G.; Lee, C.S.; Huh, Y.; Roh, C. A Rapid in Situ Colorimetric Assay for Cobalt Detection by the Naked Eye. Sensors 2016, 16, 626. [Google Scholar] [CrossRef] [PubMed]
- Alomar, T.S.; Habila, M.A.; AlMasoud, N.; Alothman, Z.A.; Sheikh, M.; Soylak, M. Biomass-derived adsorbent for dispersive solid-phase extraction of Cr (III), Fe (III), Co (II) and Ni (II) from food samples prior to ICP-MS detection. Appl. Sci. 2021, 11, 7792. [Google Scholar] [CrossRef]
- Khalil, S.; El-Sharnouby, M. Construction of a Highly Selective Membrane Sensor for the Determination of Cobalt (II) Ions. Chemosensors 2021, 9, 86. [Google Scholar] [CrossRef]
- Santos, L.B.; Assis, R.D.S.D.; Silva, U.N.; Lemos, V.A. Switchable-hydrophilicity solvent-based liquid-phase microextraction in an on-line system: Cobalt determination in food and water samples. Talanta 2022, 238, 123038. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Arnold, J. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 3, pp. 113–127. [Google Scholar]
- Stuzhin, P.A. Theoretical AM1 study of acidity of porphyrins, azaporphyrins and porphyrazines. J. Porphyr. Phthalocyanines 2003, 7, 813–832. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, S.; Senge, M.O. The good, the bad, and the ugly–controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem. Photobiol. Sci. 2018, 14, 1490–1514. [Google Scholar] [CrossRef] [PubMed]
- Redl, F.X.; Lutz, M.; Daub, J. Chemistry of Porphyrin-Appended Cellulose Strands with a Helical Structure: Spectroscopy, Electrochemistry, and in situ Circular Dichroism Spectroelectrochemistry. Chem. Eur. J. 2001, 7, 5350–5358. [Google Scholar] [CrossRef]
- Rodríguez-Morgade, M.S.; de la Torre, G.; Torres, T. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; Volume 15, pp. 125–159. [Google Scholar]
- Dmitrieva, O.A.; Ivanova, Y.B.; Semeikin, A.S.; Mamardashvili, N.Z. Fluorescence properties and quantum-chemical modeling of tert-butyl-substituted porphyrazines: Structural and ionization effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118601. [Google Scholar] [CrossRef] [PubMed]

























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fringu, I.; Anghel, D.; Fratilescu, I.; Epuran, C.; Birdeanu, M.; Fagadar-Cosma, E. Nanomaterials Based on 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine Exhibiting Bifunctional Sensitivity for Monitoring Chloramphenicol and Co2+. Biomedicines 2024, 12, 770. https://doi.org/10.3390/biomedicines12040770
Fringu I, Anghel D, Fratilescu I, Epuran C, Birdeanu M, Fagadar-Cosma E. Nanomaterials Based on 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine Exhibiting Bifunctional Sensitivity for Monitoring Chloramphenicol and Co2+. Biomedicines. 2024; 12(4):770. https://doi.org/10.3390/biomedicines12040770
Chicago/Turabian StyleFringu, Ionela, Diana Anghel, Ion Fratilescu, Camelia Epuran, Mihaela Birdeanu, and Eugenia Fagadar-Cosma. 2024. "Nanomaterials Based on 2,7,12,17-Tetra-tert-butyl-5,10,15,20-tetraaza-21H,23H-porphine Exhibiting Bifunctional Sensitivity for Monitoring Chloramphenicol and Co2+" Biomedicines 12, no. 4: 770. https://doi.org/10.3390/biomedicines12040770