Optimization of a Photobiomodulation Protocol to Improve the Cell Viability, Proliferation and Protein Expression in Osteoblasts and Periodontal Ligament Fibroblasts for Accelerated Orthodontic Treatment
Abstract
:1. Background
2. Material and Methods
2.1. Cell Culture
2.2. Cell Seeding
2.3. Photobiomodulation (PBM) Protocol
2.4. Cellular Assays
2.4.1. Metabolic Activity
2.4.2. Cell Counting
2.4.3. Alkaline Phosphatase Expression
2.4.4. Enzyme-Linked Immunosorbent Assay (ELISA) Assays
2.5. Statistical Analysis
3. Results
3.1. Initial Screening
3.2. Optimal Parameter Testing Phase
3.2.1. Metabolic Activity
3.2.2. Alkaline Phosphatase Expression
3.2.3. Osteoprotegerin (OPG) and Receptor Activator of Nuclear Factor κ-Β Ligand (RANK-L) Expression
4. Discussion
5. Conclusions
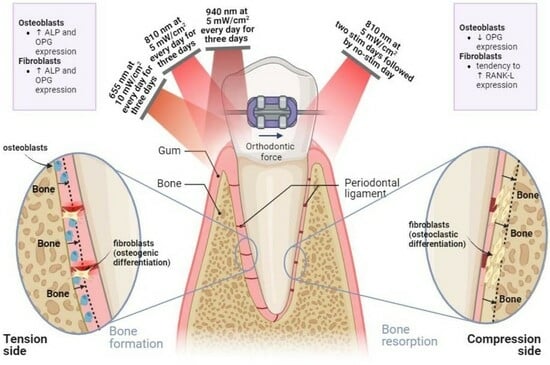
- On the tension side, daily PBM of 655 nm delivered at 10 mW/cm2, 810 nm at 5 mW/cm2 and 940 nm at 5 mW/cm2 should be applied for three consecutive days to stimulate bone formation;
- On the compression side, PBM of 810 nm at 5 mW/cm2 must be applied for two consecutive days, and the stimulation must be suspended by the third day in order to promote bone resorption phenomena;
- Low irradiation intensity levels (up to 10 mW/cm2) are enough to produce positive bioeffects on fibroblastic and osteoblastic metabolism.
6. Future Perspectives
- Longitudinal analysis may be held to ascertain the long-term responsiveness to irradiation, including protein expression patterns and safety concerns (e.g., tissue damage);
- Different stimulation periodicities must be tested (e.g., cycles of two stimulation days/one suspension day, sessions every other day, sessions every two days), as daily stimulation may not be ideal for enhancing the metabolism of certain periodontal tissues;
- The monitorization of inflammatory markers, such as IL-1β and other cytokines, under PBM may be considered, since pro-inflammatory markers modulate the activity and differentiation of periodontal ligament fibroblastic cells, which are pivotal in bone turnover mechanisms and soft tissue regeneration;
- Given the influence of external conditions, such as the biochemical environment and mechanical loads, on the effect of PBM on the expression of key bone remodeling mediators, future in vitro and animal studies should focus on investigating how the optimal PBM protocols defined here can impact the biodynamics of cells and tissues subjected to tensile and compressive forces;
- Different physical stimuli, such as light, mechanical deformations, electrical signals and others, are capable of activating and modulating distinct metabolic and signaling pathways in the periodontal tissues. In this sense, the combination of PBM with alternative therapeutic modalities (e.g., ultrasound stimulation, application of static magnetic fields) may be an interesting feature to unveil in forthcoming research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Lynch, E. Systematic Review of Orthodontic Treatment Management with Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 862–868. [Google Scholar] [CrossRef] [PubMed]
- AlShahrani, I.; Togoo, R.A.; Hosmani, J.; Alhaizaey, A. Photobiomodulation in acceleration of orthodontic tooth movement: A systematic review and meta analysis. Complement. Ther. Med. 2019, 47, 102220. [Google Scholar] [CrossRef] [PubMed]
- Meme’, L.; Gallusi, G.; Coli, G.; Strappa, E.; Bambini, F.; Sampalmieri, F. Photobiomodulation to Reduce Orthodontic Treatment Time in Adults: A Historical Prospective Study. Appl. Sci. 2022, 12, 11532. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Z.; Li, J. Efficiency of photobiomodulation on accelerating the tooth movement in the alignment phase of orthodontic treatment-A systematic review and meta-analysis. Heliyon 2023, 9, 13220. [Google Scholar] [CrossRef]
- Nimeri, G.; Kau, C.H.; Corona, R.; Shelly, J. The effect of photobiomodulation on root resorption during orthodontic treatment. Clin. Cosmet. Investig. Dent. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Crous, A.; Abrahamse, H. The Signalling Effects of Photobiomodulation on Osteoblast Proliferation, Maturation and Differentiation: A Review. Stem Cell Rev. Rep. 2021, 17, 1570–1589. [Google Scholar] [CrossRef] [PubMed]
- Grajales, M.; Ríos-Osorio, N.; Jimenez-Peña, O.; Mendez-Sanchez, J.; Sanchez-Fajardo, K.; García-Perdomo, H.A. Effectiveness of photobiomodulation with low-level lasers on the acceleration of orthodontic tooth movement: A systematic review and meta-analysis of split-mouth randomised clinical trials. Lasers Med. Sci. 2023, 38, 200. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Mylona, V.; Anagnostaki, E.; Chiniforush, N.; Barikani, H.; Lynch, E.; Grootveld, M. Photobiomodulation effects on periodontal ligament stem cells: A systematic review of in-vitro studies. Curr. Stem Cell Res. Ther. 2022, 19, 544–558. [Google Scholar] [CrossRef]
- Yong, J.; Gröger, S.; Von Bremen, J.; Marques, M.M.; Braun, A.; Chen, X.; Ruf, S.; Chen, Q. Photobiomodulation therapy assisted orthodontic tooth movement: Potential implications, challenges, and new perspectives. J. Zhejiang Univ. B 2023, 24, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Ortarzewska, M.; Jankowski, J.; Lehmann, A.; Surdacka, A. The Role of Cellular Metabolism in Maintaining the Function of the Dentine-Pulp Complex: A Narrative Review. Metabolites 2023, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.D.; Montoya Guzmán, D.; Velásquez Cujar, S.A. Effective Wavelength Range in Photobiomodulation for Tooth Movement Acceleration in Orthodontics: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2020, 38, 581–590. [Google Scholar] [CrossRef]
- Schröder, A.; Bauer, K.; Spanier, G.; Proff, P.; Wolf, M.; Kirschneck, C. Expression kinetics of human periodontal ligament fibroblasts in the early phases of orthodontic tooth movement. J. Orofac. Orthop. 2018, 79, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Yang, Y. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone Jt. Res. 2021, 8, 19–31. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Hodjat, M.; Hakimiha, N.; Akhoundi, M.S.A.; Kharazifard, M.J. In Vitro Effect of Photobiomodulation Therapy with 980 nm Diode Laser on Gene Expression of Key Regulators of Bone Remodeling by Human Periodontal Ligament Cells under Mild Orthodontic Forces. Photochem. Photobiol. 2023, 99, 1448–1455. [Google Scholar] [CrossRef]
- Asiry, M.A. Biological aspects of orthodontic tooth movement: A review of literature. Saudi J. Biol. Sci. 2018, 25, 1027–1032. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Niimi, H.; Shimohira, T.; Hatasa, M.; Katagiri, S.; Aoki, A.; Iwata, T. In vitro cytological responses against laser photobiomodulation for periodontal regeneration. Int. J. Mol. Sci. 2020, 21, 9002. [Google Scholar] [CrossRef]
- Bölükbaşı Ateş, G.; Ak Can, A.; Gülsoy, M.; Ateş, G.B.; Can, A.A.; Gülsoy, M. Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med. Sci. 2017, 32, 591–599. [Google Scholar] [CrossRef]
- Chaweewannakorn, C.; Santiwong, P.; Surarit, R.; Sritanaudomchai, H.; Chintavalakorn, R. The effect of LED photobiomodulation on the proliferation and osteoblastic differentiation of periodontal ligament stem cells: In vitro. J. World Fed. Orthod. 2021, 10, 79–85. [Google Scholar] [CrossRef]
- Hong, J.U.; Kwon, J.J.; Heo, S.C.; Shin, S.H.; Kim, H.J.; Lee, J.Y. Effects of photobiomodulation on bone remodeling in an osteoblast–osteoclast co-culture system. Lasers Med. Sci. 2022, 37, 1049–1059. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, H.; Yang, Y.; Zhou, Y.; Gu, Y.; Zhao, X.; Zhang, Y.; Zhao, Z.; Zhang, L.; Yin, J. Effects of vascular endothelial cells on osteogenic differentiation of noncontact co-cultured periodontal ligament stem cells under hypoxia. J. Periodontal Res. 2013, 48, 52–65. [Google Scholar] [CrossRef]
- Gholami, L.; Hendi, S.S.; Saidijam, M.; Mahmoudi, R.; Tarzemany, R.; Arkian, A.; Afshar, S.; Fekrazad, R. Near-infrared 940-nm diode laser photobiomodulation of inflamed periodontal ligament stem cells. Lasers Med. Sci. 2022, 37, 449–459. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Mathelié-Guinlet, Q.; Ramires, F.; Monteiro, F.; Carvalho, O.; Silva, F.S.; Resende, A.; Pinho, T. Biological alterations associated with the orthodontic treatment with conventional appliances and aligners: A systematic review of clinical and preclinical evidence. Heliyon 2024. [Google Scholar]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9, S1. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.; Carvalho, Ó.; Sousa, N.; Silva, F.S.; Sotiropoulos, I. Photobiomodulation and visual stimulation against cognitive decline and Alzheimer’s disease pathology: A systematic review. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12249. [Google Scholar] [CrossRef]
- Oliveira, S.; Andrade, R.; Hinckel, B.B.; Silva, F.; Espregueira-Mendes, J.; Carvalho, Ó.; Leal, A. In Vitro and In Vivo Effects of Light Therapy on Cartilage Regeneration for Knee Osteoarthritis: A Systematic Review. Cartilage 2021, 13 (Suppl. S2), 1700S––1719S. [Google Scholar] [CrossRef]
- Yi, J.; Xiao, J.; Li, H.; Li, Y.; Li, X.; Zhao, Z. Effectiveness of adjunctive interventions for accelerating orthodontic tooth movement: A systematic review of systematic reviews. J. Oral Rehabil. 2017, 44, 636–654. [Google Scholar] [CrossRef]
- Gama, S.K.C.; Habib, F.A.L.; de Carvalho, J.S.; Monteiro; Paraguassú, G.M.; Araújo, T.M.; Cangussú, M.C.T.; Pinheiro, A.L.B. Tooth movement after infrared laser phototherapy: Clinical study in rodents. Photomed. Laser Surg. 2010, 28 (Suppl. S2), S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in low level light therapy (LLLT) for dentistry. Dent. Mater. 2014, 30, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, A.; Horodynski, M.; Fusco, R.; Palaia, G.; Polimeni, A.; Romeo, U.; Barbato, E.; Galluccio, G. Photobiomodulation therapy on orthodontic movement: Analysis of preliminary studies with a new protocol. Int. J. Environ. Res. Public Health 2020, 17, 3547. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Sadatmansouri, S.; Sodeif, F.; Jalalishirazi, F.; Chiniforush, N. Photobiomodulation Effect of Different Diode Wavelengths on the Proliferation of Human Gingival Fibroblast Cells. Photochem. Photobiol. 2021, 97, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Incerti-Parenti, S.; Cepollaro, S.; Checchi, L.; Fini, M. Photobiomodulation with low-level diode laser promotes osteoblast migration in an in vitro micro wound model. J. Biomed. Opt. 2015, 20, 078002. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Shimizu, N.; Abiko, Y. Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg. Med. 1997, 21, 456–463. [Google Scholar] [CrossRef]
- Mayahara, K.; Yamaguchi, A.; Sakaguchi, M.; Igarashi, Y.Y.; Shimizu, N. Effect of Ga-Al-As laser irradiation on COX-2 and cPLA2α expression in compressed human periodontal ligament cells. Lasers Surg. Med. 2010, 42, 489–493. [Google Scholar] [CrossRef]
- Chang, Y.C.; Zhao, J.H. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust. Dent. J. 2011, 56, 365–371. [Google Scholar] [CrossRef]
- Jawad, M.M.; Husein, A.; Azlina, A.; Alam, M.K.; Hassan, R.; Shaari, R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J. Biomed. Opt. 2013, 18, 128001. [Google Scholar] [CrossRef]
- Murakami, Y.; Kojima, T.; Nagasawa, T.; Kobayashi, H.; Ishikawa, I. Novel Isolation of Alkaline Phosphatase-Positive Subpopulation from Periodontal Ligament Fibroblasts. J. Periodontol. 2003, 74, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Deng, T.; Mo, F.; Deng, B.; Lam, W.; Deng, P.; Zhang, X.; Liu, S. Low-Intensity Pulsed Laser Irradiation Affects RANKL and OPG mRNA Expression in Rat Calvarial Cells. Photomed. Laser Surg. 2009, 27, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Abe, T.; Medina, C.C.C.C.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Examination of the effect of the combined use of Nd: Yag laser irradiation and mechanical force loading on bone metabolism using cultured human osteoblasts. J. Lasers Med. Sci. 2020, 11, 138–143. [Google Scholar] [CrossRef] [PubMed]







| Wavelength (nm) | Power Density (mW/cm2) | Duration (min) | Number of Sessions | Follow-Ups | |
|---|---|---|---|---|---|
| Initial screening | 655 810 940 | 5 10 | 3 | 1 | 1, 24 and 72 h (after stimulation) |
| Optimal parameter testing phase | 3 | 1, 2 and 3 days (after each stimulation) |
| Metabolic Activity # | ALP Expression # | OPG Expression # | RANK-L Expression # | ||
|---|---|---|---|---|---|
| Periodontal ligament fibroblasts | ↑ |
|
|
|
|
| ↓ | - | - | - | - | |
| Osteoblasts | ↑ |
|
|
| - |
| ↓ | - | - |
| - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.; Monteiro, F.; Oliveira, S.; Costa, I.; Catarino, S.O.; Carvalho, Ó.; Padrão, J.; Zille, A.; Pinho, T.; Silva, F.S. Optimization of a Photobiomodulation Protocol to Improve the Cell Viability, Proliferation and Protein Expression in Osteoblasts and Periodontal Ligament Fibroblasts for Accelerated Orthodontic Treatment. Biomedicines 2024, 12, 180. https://doi.org/10.3390/biomedicines12010180
Gonçalves A, Monteiro F, Oliveira S, Costa I, Catarino SO, Carvalho Ó, Padrão J, Zille A, Pinho T, Silva FS. Optimization of a Photobiomodulation Protocol to Improve the Cell Viability, Proliferation and Protein Expression in Osteoblasts and Periodontal Ligament Fibroblasts for Accelerated Orthodontic Treatment. Biomedicines. 2024; 12(1):180. https://doi.org/10.3390/biomedicines12010180
Chicago/Turabian StyleGonçalves, Aline, Francisca Monteiro, Sofia Oliveira, Inês Costa, Susana O. Catarino, Óscar Carvalho, Jorge Padrão, Andrea Zille, Teresa Pinho, and Filipe S. Silva. 2024. "Optimization of a Photobiomodulation Protocol to Improve the Cell Viability, Proliferation and Protein Expression in Osteoblasts and Periodontal Ligament Fibroblasts for Accelerated Orthodontic Treatment" Biomedicines 12, no. 1: 180. https://doi.org/10.3390/biomedicines12010180