TAS2R38 Bitter Taste Receptor Polymorphisms in Patients with Chronic Rhinosinusitis with Nasal Polyps Preliminary Data in Polish Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Enrolment and Clinical Data Collection
2.2. DNA Genotyping for TAS2R38 Polymorphism
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Garcia-Sanchez, A.; Estravis, M.; Gil-Melcón, M.; Isidoro-Garcia, M.; Sanz, C.; Davila, I. Genetics and Epigenetics of Nasal Polyposis: A Systematic Review. J. Investig. Allergol. Clin. Immunol. 2021, 31, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Purnell, P.R.; Addicks, B.L.; Zalzal, H.G.; Shapiro, S.; Wen, S.; Ramadan, H.H.; Setola, V.; Siderovski, D.P. Single Nucleotide Polymorphisms in Chemosensory Pathway Genes GNB3, TAS2R19, and TAS2R38 Are Associated with Chronic Rhinosinusitis. Int. Arch. Allergy Immunol. 2019, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mfuna Endam, L.; Filali-Mouhim, A.; Boisvert, P.; Boulet, L.P.; Bossé, Y.; Desrosiers, M. Genetic variations in taste receptors are associated with chronic rhinosinusitis: A replication study. Int. Forum Allergy Rhinol. 2014, 4, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Carey, R.M.; Lee, R.J.; Cohen, N.A. Taste Receptors in Upper Airway Immunity. Adv. Otorhinolaryngol. 2016, 79, 91–102. [Google Scholar] [CrossRef]
- Rowan, N.R.; Soler, Z.M.; Othieno, F.; Storck, K.A.; Smith, T.L.; Schlosser, R.J. Impact of bitter taste receptor phenotype upon clinical presentation in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 1013–1020. [Google Scholar] [CrossRef]
- Kim, U.; Wooding, S.; Ricci, D.; Jorde, L.B.; Drayna, D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum. Mutat. 2005, 26, 199–204. [Google Scholar] [CrossRef]
- Drayna, D. Human Taste Genetics. Annu. Rev. Genomics Hum. Genet. 2005, 6, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Howland, T.J.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Lee, R.J.; Cohen, N.A. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int. Forum Allergy Rhinol. 2013, 3, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2014, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Farquhar, D.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Morris, S.A.; Owens, D.; Mansfield, C.; Lysenko, A.; Lee, R.J.; et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Deshaware, S.; Singhal, R. Genetic variation in bitter taste receptor gene TAS2R38, PROP taster status and their association with body mass index and food preferences in Indian population. Gene 2017, 627, 363–368. [Google Scholar] [CrossRef]
- Sandell, M.; Hoppu, U.; Mikkilä, V.; Mononen, N.; Kähönen, M.; Männistö, S.; Rönnemaa, T.; Viikari, J.; Lehtimäki, T.; Raitakari, O.T. Genetic variation in the hTAS2R38 taste receptor and food consumption among Finnish adults. Genes. Nutr. 2014, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Dżaman, K.; Zagor, M.; Stachowiak, M.; Bielińska, B.; Sarnowska, E.; Krzeski, A.; Kantor, I. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngol. Pol. 2016, 70, 13–18. [Google Scholar] [CrossRef]
- Shivaprasad, H.S.; Chaithra, P.T.; Kavitha, P.; Malini, S.S. Role of phenylthiocarbamide as a genetic marker in predicting the predisposition of disease traits in humans. J. Nat. Sci. Biol. Med. 2012, 3, 43–47. [Google Scholar] [CrossRef]
- Guo, S.W.; Reed, D.R. The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 2001, 28, 111–142. [Google Scholar] [CrossRef]
- Kim, U.K.; Drayna, D. Genetics of individual differences in bitter taste perception: Lessons from the PTC gene. Clin. Genet. 2005, 67, 275–280. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Kennedy, D.W. Staging for rhinosinusitis. Otolaryngol. Head. Neck Surg. 1997, 117 (Suppl. 3), S35–S40. [Google Scholar] [CrossRef]
- Hopkins, C.; Browne, J.P.; Slack, R.; Lund, V.; Brown, P. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngol.—Head Neck Surg. 2007, 137, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Psychophysical dissection of genotype effects on human bitter perception. Chem. Senses. 2011, 36, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, E.M.; Konecki, T.; Pietrusiński, M.; Borowiec, M.; Jabłonowski, Z. MicroRNAs Which Can Prognosticate Aggressiveness of Bladder Cancer. Cancers 2019, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Gavazaj, F.Q.; Mikerezi, I.I.; Morina, V.H.; Cakaj, F.A.; Maloku, E.B.; Gavazaj, B.B.; Kastrati, D.S.; Duriqi-Maloku, B.A. Optimization of DNA concentration to amplify short tandem repeats of human genomic DNA. Bosn. J. Basic. Med. Sci. 2012, 12, 236–239. [Google Scholar] [CrossRef]
- Malik, B.; Elkaddi, N.; Turkistani, J.; Spielman, A.I.; Ozdener, M.H. Mammalian Taste Cells Express Functional Olfactory Receptors. Chem. Senses. 2019, 44, 289–301. [Google Scholar] [CrossRef]
- Chen, J.H.; Song, C.I.; Hura, N.; Saraswathula, A.; Seal, S.M.; Lane, A.P.; Rowan, N.R. Taste receptors in chronic rhinosinusitus, what is the evidence? A systematic review. Int. Forum Allergy Rhinol. 2022, 12, 917–934. [Google Scholar] [CrossRef]
- Risso, D.S.; Mezzavilla, M.; Pagani, L.; Robino, A.; Morini, G.; Tofanelli, S.; Carrai, M.; Campa, D.; Barale, R.; Caradonna, F.; et al. Global diversity in the TAS2R38 bitter taste receptor: Revisiting a classic evolutionary PROPosal. Sci. Rep. 2016, 6, 25506. [Google Scholar] [CrossRef]
- Carrai, M.; Steinke, V.; Vodicka, P.; Pardini, B.; Rahner, N.; Holinski-Feder, E.; Morak, M.; Schackert, H.K.; Görgens, H.; Stemmler, S.; et al. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: A case-control study in two independent populations of Caucasian origin. PLoS ONE 2011, 6, e20464. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Errigo, A.; Crnjar, R.; Pes, G.M.; Tomassini Barbarossa, I. TAS2R38 bitter taste receptor and attainment of exceptional longevity. Sci. Rep. 2019, 9, 18047. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Roman, S.; Martinez-Lopez, E.; Gonzalez-Aldaco, K.; Ojeda-Granados, C.; Sepulveda-Villegas, M.; Panduro, A. Association of a novel TAS2R38 haplotype with alcohol intake among Mexican-Mestizo population. Ann. Hepatol. 2015, 14, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Boxer, E.E.; Garneau, N.L. Rare haplotypes of the gene TAS2R38 confer bitter taste sensitivity in humans. SpringerPlus 2015, 4, 505. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H. Variation in the TAS2R38 Bitterness Receptor Gene Was Associated with Food Consumption and Obesity Risk in Koreans. Nutrients 2019, 11, 1973. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.; Choi, I.J.; Kim, Y.W.; Ryu, K.W.; Kim, J. Genetic Variation in the TAS2R38 Bitter Taste Receptor and Gastric Cancer Risk in Koreans. Sci. Rep. 2016, 6, 26904. [Google Scholar] [CrossRef]
- Piatti, G.; Ambrosetti, U.; Aldè, M.; Girotto, G.; Concas, M.P.; Torretta, S. Chronic Rhinosinusitis: T2r38 Genotyping and Nasal Cytology in Primary Ciliary Dyskinesia. Laryngoscope 2022, 133, 248–254. [Google Scholar] [CrossRef]
- Adappa, N.D.; Workman, A.D.; Hadjiliadis, D.; Dorgan, D.J.; Frame, D.; Brooks, S.; Doghramji, L.; Palmer, J.N.; Mansfield, C.; Reed, D.R.; et al. T2R38 genotype is correlated with sinonasal quality of life in homozygous ΔF508 cystic fibrosis patients. Int. Forum Allergy Rhinol. 2016, 6, 356–361. [Google Scholar] [CrossRef]
- Cantone, E.; Negri, R.; Roscetto, E.; Grassia, R.; Catania, M.R.; Capasso, P.; Maffei, M.; Soriano, A.A.; Leone, C.A.; Iengo, M.; et al. In Vivo Biofilm Formation, Gram-Negative Infections and TAS2R38 Polymorphisms in CRSw NP Patients. Laryngoscope 2018, 128, E339–E345. [Google Scholar] [CrossRef]
- Adappa, N.D.; Truesdale, C.M.; Workman, A.D.; Doghramji, L.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Cowart, B.J.; Cohen, N.A. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2016, 6, 783–791. [Google Scholar] [CrossRef]
- Cohen, N.A. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope 2017, 127, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Grossi, S.; Montrasio, G.; Binelli, G.; Cinquetti, R.; Simmen, D.; Castelnuovo, P.; Campomenosi, P. TAS2R38 taste receptor gene and chronic rhinosinusitis: New data from an Italian population. BMC Med. Genet. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Melis, M.; Sarchioto, M.; Melis, M.; Melis, M.; Morelli, M.; Tomassini Barbarossa, I. 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov. Disord. 2018, 33, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]



| Study Group (N = 106) | ||
|---|---|---|
| Gender (%) | F | 44 (41.51%) |
| M | 62 (58.49%) | |
| Age (SD) | 46.82 (14.02) | |
| Weight (SD) | 81.29 (17.15) | |
| Height (SD) | 172.89 (9.23) | |
| BMI (SD) | 27.12 (4.97) | |
| PTC tasting (%) | 0 | 36 (33.96%) |
| 1 | 70 (66.04%) | |
| Age of the first symptoms (%) | <16 y.o. 16–40 y.o. >40 y.o. | 23 (21.7%) 51 (48.11%) 32 (30.2%) |
| Asthma (%) | 0 1 | 86 (81.13%) 20 (18.87%) |
| Seasonal allergies (%) | 25 (23.58%) | |
| Year-round allergies (%) | 27 (25.47%) | |
| Tabaco usage (%) | 18 (16.98%) | |
| VAS score (%) | 0–3 | 47 (44.34%) |
| 4–7 | 35 (33.02%) | |
| 8–10 | 24 (22.64%) | |
| Median SNOT-22 score (SD) | 32 (9.037) | |
| Median Lund–Kennedy score (SD) | 3 (1.14) | |
| Median Lund–Mackay score (SD) | 9 (5.474) | |
| SG vs. CG | ||||
|---|---|---|---|---|
| Haplotype | Control Group N (%) | Study Group N (%) | OR (95% CI) | p-Value |
| PAV/PAV | 100 (22.8) | 13 (12.3) | 1.0 * | |
| AVI/AVI | 104 (23.7) | 33 (31.1) | 2.44 (1.21–4.90) | p = 0.01 |
| PAV/AVI | 234 (53.5) | 60 (56.6) | 1.97 (1.03–3.75) | p = 0.04 |
| AVI/AVI; PAV/AVI | 338 (77) | 93 (88) | 2.11 (1.136–3.941) | p = 0.02 |
| AVI | 443 (50.6) | 126 (59.4) | 1.43 (1.05–1.94) | |
| PAV | 433 (49.4) | 86 (40.6) | 1.0 * | p = 0.02 |
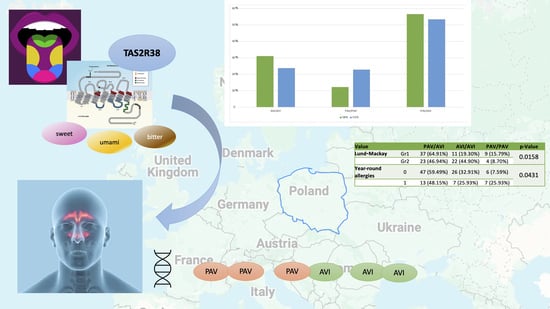
| Value | PAV/AVI | AVI/AVI | PAV/PAV | p-Value | |
|---|---|---|---|---|---|
| VAS | 0–3 | 26 (55.32%) | 12 (25.53%) | 9 (19.15%) | 0.3528 |
| 4–7 | 20 (57.14%) | 12 (34.29%) | 3 (8.57%) | ||
| 8–10 | 14 (58.33%) | 9 (37.50%) | 1 (4.17%) | ||
| SNOT-22 | Gr1 | 35 (58.33%) | 17 (28.33%) | 8 (13.33%) | 0.7596 |
| Gr2 | 25 (54.35%) | 16 (34.78%) | 5 (10.87%) | ||
| Lund–Kennedy | Gr1 | 42 (56.76%) | 21 (28.38%) | 11 (14.86%) | 0.3772 |
| Gr2 | 18 (56.25%) | 12 (37.50%) | 2 (6.25%) | ||
| Lund–Mackay | Gr1 | 37 (64.91%) | 11 (19.30%) | 9 (15.79%) | 0.0158 |
| Gr2 | 23 (46.94%) | 22 (44.90%) | 4 (8.70%) | ||
| PTC | 0 | 24 (66.67%) | 7 (19.44%) | 5 (13.89%) | 0.1751 |
| 1 | 36 (51.43%) | 26 (37.14%) | 8 (11.43%) | ||
| Asthma | 0 | 52 (60.47%) | 23 (16.74%) | 11 (12.79%) | 0.1273 |
| 1 | 8 (40.00%) | 10 (50.00%) | 2 (10.00%) | ||
| Seasonal allergies | 0 | 46 (56.79%) | 26 (32.10%) | 9 (11.11%) | 0.7876 |
| 1 | 14 (56.00%) | 7 (28.00%) | 4 (16.00%) | ||
| Year-round allergies | 0 | 47 (59.49%) | 26 (32.91%) | 6 (7.59%) | 0.0431 |
| 1 | 13 (48.15%) | 7 (25.93%) | 7 (25.93%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeruzal-Świątecka, J.; Borkowska, E.M.; Borkowska, M.; Pietruszewska, W. TAS2R38 Bitter Taste Receptor Polymorphisms in Patients with Chronic Rhinosinusitis with Nasal Polyps Preliminary Data in Polish Population. Biomedicines 2024, 12, 168. https://doi.org/10.3390/biomedicines12010168
Jeruzal-Świątecka J, Borkowska EM, Borkowska M, Pietruszewska W. TAS2R38 Bitter Taste Receptor Polymorphisms in Patients with Chronic Rhinosinusitis with Nasal Polyps Preliminary Data in Polish Population. Biomedicines. 2024; 12(1):168. https://doi.org/10.3390/biomedicines12010168
Chicago/Turabian StyleJeruzal-Świątecka, Joanna, Edyta Marta Borkowska, Martyna Borkowska, and Wioletta Pietruszewska. 2024. "TAS2R38 Bitter Taste Receptor Polymorphisms in Patients with Chronic Rhinosinusitis with Nasal Polyps Preliminary Data in Polish Population" Biomedicines 12, no. 1: 168. https://doi.org/10.3390/biomedicines12010168