The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness
Abstract
:1. Introduction
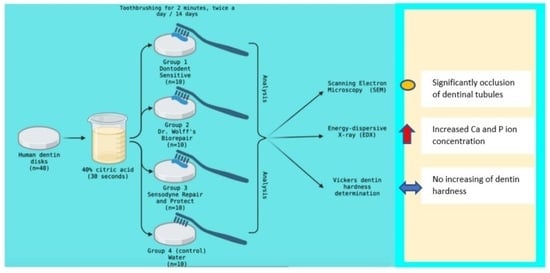
2. Materials and Methods
2.1. Teeth Collection and Sample Preparation
2.2. Dentinal Tubules Occlusion by SEM Evaluation
2.3. Mineral Evaluation by Energy-Dispersive X-ray (EDX) Analysis
2.4. Dentin Hardness Evaluation
2.5. Statistical Analysis
3. Results
3.1. SEM Evaluation Results
3.2. Energy-Dispersive X-ray (EDX) Evaluation Results
3.3. Dentin Hardness Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lussi, A. Dental Erosion: From Diagnosis to Therapy; Karger Medical and Scientific Publishers: Bern, Switzerland, 2006; p. 219. [Google Scholar]
- Cunha-Cruz, J.; Wataha, J.C.; Heaton, L.J.; Rothen, M.; Sobieraj, M.; Scott, J.; Berg, J. The prevalence of dentin hypersensitivity in general dental practices in the northwest United States. J. Am. Dent. Assoc. 2013, 144, 288–296. [Google Scholar] [CrossRef]
- Yadav, B.K.; Jain, A.; Rai, A.; Jain, M. Dentine Hypersensitivity: A Review of its Management Strategies. J. Int. Oral Health 2015, 7, 137–143. [Google Scholar]
- Liu, X.X.; Tenenbaum, H.C.; Wilder, R.S.; Quock, R.; Hewlett, E.R.; Ren, Y.F. Pathogenesis, diagnosis and management of dentin hypersensitivity: An evidence-based overview for dental practitioners. BMC Oral Health. 2020, 20, 220. [Google Scholar] [CrossRef]
- Gillam, D.G. Dentine Hypersensitivity: Advances in Diagnosis, Management, and Treatment; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–190. [Google Scholar]
- Eder, A.; Faigenblum, M. Tooth Wear: An Authoritative Reference for Dental Professionals and Students, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2022; p. 92. [Google Scholar]
- Borges, A.; Barcellos, D.; Gomes, C. Dentin Hypersensitivity-Etiology, Treatment Possibilities and Other Related Factors: A Literature review. World J. Dent. 2012, 3, 60–67. [Google Scholar]
- Seligman, D.A.; Pullinger, A.G.; Solberg, W.K. The prevalence of dental attrition and its association with factors of age, gender, occlusion, and TMJ symptomatology. J. Dent. Res. 1988, 67, 1323–1333. [Google Scholar] [CrossRef]
- Grippo, J.O.; Simring, M.; Schreiner, S. Attrition, Attrition, abrasion, corrosion and abfraction revisited: A new perspective on tooth surface lesions. J. Am. Dent. Assoc. 2004, 135, 1109–1118. [Google Scholar] [CrossRef]
- West, N.X.; Seong, J.; Hellin, N.; Macdonald, E.L.; Jones, S.B.; Creeth, J.E. Assessment of tubule occlusion properties of an experimental stannous fluoride toothpaste: A randomised clinical in situ study. J. Dent. 2018, 76, 125–131. [Google Scholar] [CrossRef]
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. 2003, 134, 220–225. [Google Scholar] [CrossRef]
- Baker, P.; Spedding, C. The aetiology of gingival recession. Dent. Update 2002, 29, 59–62. [Google Scholar] [CrossRef]
- Lussi, A.; Ganss, C. Erosive Tooth Wear: From Diagnosis to Therapy; Karger Medical and Scientific Publishers: Bern, Switzerland, 2014; p. 284. [Google Scholar]
- Gillam, D.G.; Orchardson, R. Advances in the treatment of root dentin sensitivity: Mechanisms and treatment principles. Endod. Topics 2006, 13, 13–33. [Google Scholar] [CrossRef]
- Wennstrom, J.L. Mucogingival therapy. Ann. Periodontol. 1996, 1, 671–701. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Sahrmann, P. Current management of dentin hypersensitivity. Clin. Oral. Investig. 2013, 17, 55. [Google Scholar] [CrossRef]
- Kazemi, R.B.; Sen, B.H.; Spångberg, L.S.W. Permeability changes of dentine treated with titanium tetrafluoride. J. Dent. 1999, 27, 531–538. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentin permeability, dentin sensitivity and treatment through tubule occlusion. J. Endod. 1986, 12, 465–474. [Google Scholar] [CrossRef]
- Prati, C.; Chersoni, S.; Lucchese, A.; Pashley, D.H.; Mongiorgi, R. Dentin permeability after toothbrushing with different toothpastes. Am. J. Dent. 1999, 12, 190–193. [Google Scholar]
- Prati, C.; Venturi, L.; Valdrè, G.; Mongiorgi, R. Dentin morphology and permeability after brushing with different toothpastes in presence and absence of smear layer. J. Periodontol. 2002, 73, 183–190. [Google Scholar] [CrossRef]
- Arrais, C.A.; Micheloni, C.D.; Giannini, M.; Chan, D.C. Occluding effect of dentifrices on dentinal tubules. J. Dent. 2003, 31, 577–584. [Google Scholar] [CrossRef]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef]
- Öncü, E.; Karabekiroğlu, S.; Ünlü, N. Effects of different desensitizers and lasers on dentine tubules: An in-vitro analysis. Microsc. Res. Tech. 2017, 80, 737–744. [Google Scholar] [CrossRef]
- Stoleriu, S.; Pancu, G.; Ghiorghe, A.; Sincar, D.C.; Solomon, S.; Andrian, S.; Iovan, G. Evaluation of dentinal changes following application of three different desensitizing agents. Rev. Chim. 2017, 68, 1573–1577. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sa, Y.; Sauro, S.; Chen, H.; Xing, W.; Ma, X.; Jiang, T.; Wang, Y. Effect of desensitising toothpastes on dentinal tubule occlusion: A dentine permeability measurement and SEM in vitro study. J. Dent. 2010, 38, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Mohammadi, Y.; Rezaei-Soufi, L.; Farmany, A. Occlusion effects of bioactive glass and hydroxyapatite on dentinal tubules: A systematic review. Clin. Oral Investig. 2022, 26, 6061–6078. [Google Scholar] [CrossRef] [PubMed]
- Kulal, R.; Jayanti, I.; Sambashivaiah, S.; Bilchodmath, S. An in-vitro comparison of nano hydroxyapatite, novamin and proargin desensitizing toothpastes—A SEM study. J. Clin. Diagn. Res. 2016, 10, ZC51–ZC54. [Google Scholar] [CrossRef] [PubMed]
- Scott, R. NovaMin® Technology. J. Clin. Dent. 2010, 21, 59–60. [Google Scholar]
- Greenspan, D.C. NovaMin® and tooth sensitivity—An overview. J. Clin. Dent. 2010, 21, 61–65. [Google Scholar]
- Forsback, A.P.; Areva, S.; Salonen, J.I. Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol. Scand. 2004, 62, 14. [Google Scholar] [CrossRef]
- Mahmoodi, B.; Goggin, P.; Fowler, C.; Cook, R.B. Quantitative assessment of dentine mineralization and tubule occlusion by NovaMin and stannous fluoride using serial block face scanning electron microscopy. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 717–722. [Google Scholar] [CrossRef]
- Shah, S.; Shivakumar, A.T.; Khot, O.; Patil, C.; Hosmani, N. Efficacy of NovaMin- and Pro-Argin-Containing Desensitizing Dentifrices on Occlusion of Dentinal Tubules. Dent. Hypotheses 2017, 8, 104–109. [Google Scholar] [CrossRef]
- Gopinath, N.M.; John, J.; Nagappan, N.; Prabhu, S.; Kumar, E.S. Evaluation of Dentifrice Containing Nano-hydroxyapatite for Dentinal Hypersensitivity: A Randomized Controlled Trial. J. Int. Oral Health 2015, 7, 118–122. [Google Scholar]
- Poggio, C.; Lombardini, M.; Vigorelli, P.; Colombo, M.; Chiesa, M. The role of different toothpastes on preventing dentin erosion: An SEM and AFM study. Scanning 2014, 36, 301–310. [Google Scholar] [CrossRef]
- Arnold, W.H.; Prange, M.; Naumova, E.A. Effectiveness of various toothpastes on dentine tubule occlusion. J. Dent. 2015, 43, 440–449. [Google Scholar] [CrossRef]
- Pei, D.; Meng, Y.; Li, Y.; Liu, J.; Lu, Y. Influence of nano-hydroxyapatite containing desensitizing toothpastes on the sealing ability of dentinal tubules and bonding performance of self-etch adhesives. J. Mech. Behav. Biomed. Mater. 2019, 91, 38–44. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciù, M. Stannous Fluoride Effects on Enamel: A Systematic Review. Biomimetics 2020, 5, 41. [Google Scholar] [CrossRef]
- Naumova, E.A.; Gaengler, P.; Zimmer, S.; Arnold, W.H. Influence of individual saliva secretion on fluoride bioavailability. Open Dent. J. 2010, 4, 185–190. [Google Scholar] [CrossRef]
- Burwell, A.; Jennings, D.; Greenspan, D.C. NovaMin and dentin hypersensitivity--in vitro evidence of efficacy. J. Clin. Dent. 2010, 21, 66–71. [Google Scholar]
- Cohen, S.R.; Apter, N.; Jesse, S.; Kalinin, S.; Barlam, D.; Peretz, A.I.; Ziskind, D.; Wagner, H.D. AFM investigation of mechanical properties of dentin. Isr. J. Chem. 2008, 48, 65–72. [Google Scholar] [CrossRef]


| Materials’ Brand Name | Manufacturer | Composition |
|---|---|---|
| Dontodent Sensitive | DM Drogeria Markt, Karlsruhe, Germany | Hydroxyapatite, Sodium Fluoride, Tetrapotassium pyrophosphate, Aqua, Sorbitol, Propylene Glycol, Glycerin, Silica, Aroma, Cellulose Gum, Sodium C14–16 Olefin Sulfonate, Sodium Cocoyl Isethionate, Sodium Saccharin, Menthol, Eucalyptol, Limonene, CI 77891 |
| Dr. Wolff’s Biorepair | Dr. Kurt Wolff GmbH & Co. KG, Bielefeld, Germany | Zinc Hydroxyapatite, Aqua, Hydrated Silica, Glycerin, Sorbitol, Silica, Aroma, Cellulose Gum, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl Taurate, Tetrapotassium Pyrophosphate, Zinc Pca, Sodium Saccharin, Phenoxyethanol, Benzyl Alcohol, Propylparaben, Methylparaben, Citric Acid, Sodium Benzoate. |
| Sensodyne Repair and Protect | GlaxoSmithKline, Brentford, Middlesex, UK | Calcium sodium phosphosilicate, Stannous fluoride, Glycerin, PEG-8, hydrated silica, pentasodium triphosphate, sodium lauryl sulfate, flavour, titanium dioxide, polyacrylic acid, cocamidopropyl betaine, sodium saccharin |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Mean score value ± SD | 3.60 ± 0.69 | 2.20 ± 0.91 | 2.30 ± 1.16 | 5.00 ± 0.00 |
| Group | Compared With | Standard Error | Significance |
|---|---|---|---|
| Group 1 (DS) | Group 2 (DWB) | 5.103 | 0.216 |
| Group 3 (SRP) | 5.103 | 0.321 | |
| Group 4 (C) | 5.103 | 0.048 | |
| Group 2 (DWB) | Group 3 (SRP) | 5.103 | 1.000 |
| Group 4 (C) | 5.103 | 0.000 | |
| Group 3 (SRP) | Group 4 (C) | 5.103 | 0.000 |
| Mean Value of Ion Concentration (wt%) ± Standard Deviation | ||||
|---|---|---|---|---|
| Ion | Group 1 | Group 2 | Group 3 | Group 4 |
| Calcium | 23.657 ± 5.016 A | 20.352 ±7.373 C | 24.608 ± 3.738 A | 17.417 ± 2.381 B |
| Phosphorous | 13.320 ± 3.323 A | 11.351 ± 4.979 C | 13.202 ± 2.910 A | 8.983 ± 1.919 B |
| Carbon | 14.135 ± 6.920 A | 16.456 ± 9.109 A | 11.910 ± 7.410 A | 35.212 ± 3.712 B |
| Oxygen | 46.057 ±1.502 A | 48.137 ± 4.631 A | 47.581 ± 2.894 A | 38.089 ± 2.040 B |
| Silicon | 2.828 ± 0.838 A | 3.702 ± 1.945 A | 2.696 ± 0.980 A | 0.296 ± 0.200 B |
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Mean hardness value ± SD | 243.033 ± 100.147 A | 327.382 ± 376.653 A | 260.299 ± 157.697 A | 225.803 ± 89.934 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bologa, E.; Stoleriu, S.; Nica, I.; Tărăboanță, I.; Georgescu, A.; Matei, R.I.; Andrian, S. The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness. Biomedicines 2023, 11, 2464. https://doi.org/10.3390/biomedicines11092464
Bologa E, Stoleriu S, Nica I, Tărăboanță I, Georgescu A, Matei RI, Andrian S. The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness. Biomedicines. 2023; 11(9):2464. https://doi.org/10.3390/biomedicines11092464
Chicago/Turabian StyleBologa, Emilia, Simona Stoleriu, Irina Nica, Ionuț Tărăboanță, Andrei Georgescu, Ruxandra Ilinca Matei, and Sorin Andrian. 2023. "The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness" Biomedicines 11, no. 9: 2464. https://doi.org/10.3390/biomedicines11092464