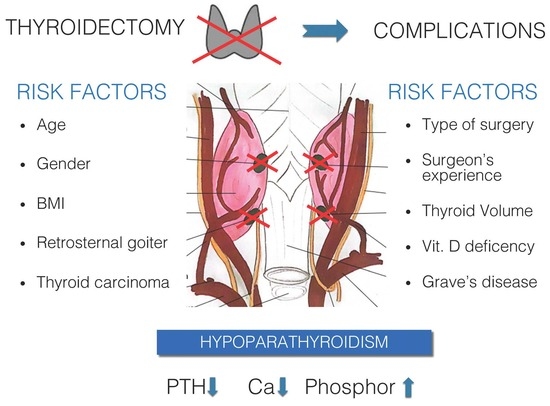
Risk Factors for Calcium-Phosphate Disorders after Thyroid Surgery
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
- (1)
- Demographics: age, gender, BMI;
- (2)
- Dependent on thyroid disease: clinical diagnosis, coexistence of autoimmune diseases (positive anti-TPO, anti-TG or TRAB antibodies), type of focal thyroid lesions: single vs. multiple vs. parenchymal goiter, presence of retrosternal goiter, tracheal displacement or narrowing;
- (3)
- Related to surgical treatment: type of surgery: primary vs. secondary, extent of thyroid surgery: total vs. partial surgery;
- (4)
- Human factor: operator experience (up to 50 thyroidectomy/year, more than 50 thyroidectomy/year);
- (5)
- Vitamin D deficiency (diagnosed when the concentration 25-hydroxyvitamin D in the blood was lower than n < 30 nmol/L) was considered a risk factor for complications, and changes in Ca, P and PTH levels before vs. after surgical treatment were analyzed.
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Statistical Analysis
3. Results
3.1. Risk Factors vs. Postoperative Hypoparathyroidism
3.2. Risk Factors vs. Hypocalcemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Slycke, S.; Van Den Heede, K.; Bruggeman, N.; Vermeersch, H.; Brusselaers, N. Risk factors for postoperative morbidity after thyroid surgery in a PROSPECTIVE cohort of 1500 patients. Int. J. Surg. 2021, 88, 105922. [Google Scholar] [CrossRef] [PubMed]
- Główny Urząd Statystyczny. Stan Zdrowia Ludności Polski w 2019 r. Available online: https://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/stan-zdrowia-ludnosci-polski-w-2019-r-,26,1.html (accessed on 12 December 2021).
- Wojciechowska, U.; Didkowska, J. Zachorowania i Zgony na Nowotwory Złośliwe w Polsce. Krajowy Rejestr Nowotworów, Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie—Państwowy Instytut Badawczy. Available online: http://onkologia.org.pl/raporty/ (accessed on 23 December 2021).
- Choroby Tarczycy u Polaków. Statystyki, Objawy, Leczenie. Available online: https://zdrowie.wprost.pl/medycyna/choroby/10398657/choroby-tarczycy-u-polakow-statystyki-objawy-leczenie.html (accessed on 15 October 2022).
- Orloff, L.A.; Wiseman, S.M.; Bernet, V.J.; Fahey, T.J., 3rd; Shaha, A.R.; Shindo, M.L.; Snyder, S.K.; Stack, B.C., Jr.; Sunwoo, J.B.; Wang, M.B. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid 2018, 28, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Bilezikian, J.P.; Shoback, D.; Bouillon, R.; Clarke, B.L.; Thakker, R.V.; Khan, A.A.; Potts, J.T., Jr. Management of Hypoparathyroidism: Summary Statement and Guidelines. J. Clin. Endocrinol. Metab. 2016, 101, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Rejnmark, L.; Marcocci, C.; Shoback, D.M.; Sitges-Serra, A.; van Biesen, W.; Dekkers, O.M. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 2015, 173, G1–G20. [Google Scholar] [CrossRef] [PubMed]
- Edafe, O.; Antakia, R.; Laskar, N.; Uttley, L.; Balasubramanian, S.P. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 2014, 101, 307–320. [Google Scholar] [CrossRef]
- Asari, R.; Passler, C.; Kaczirek, K.; Scheuba, C.; Niederle, B. Hypoparathyroidism after total thyroidectomy: A prospective study. Arch. Surg. 2008, 143, 132–137. [Google Scholar] [CrossRef]
- Bai, B.; Chen, Z.; Chen, W. Risk factors and outcomes of incidental parathyroidectomy in thyroidectomy: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0207088. [Google Scholar] [CrossRef]
- Ru, Z.; Mingliang, W.; Maofei, W.; Qiaofeng, C.; Jianming, Y. Analysis of Risk Factors for Hypoparathyroidism After Total Thyroidectomy. Front. Surg. 2021, 8, 668498. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Carsote, M.; Andronic, O.; Bolocan, A. Post-thyroidectomy Hypocalcemia—Risk Factors and Management. Chirurgia 2019, 114, 564–570. [Google Scholar] [CrossRef]
- Falch, C.; Hornig, J.; Senne, M.; Braun, M.; Konigsrainer, A.; Kirschniak, A.; Muller, S. Factors predicting hypocalcemia after total thyroidectomy—A retrospective cohort analysis. Int. J. Surg. 2018, 55, 46–50. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, W.; Wang, Z.; Dong, W.; He, L.; Zhang, T.; Zhang, H. A Meta-Analysis of Risk Factors for Transient and Permanent Hypocalcemia After Total Thyroidectomy. Front. Oncol. 2021, 10, 614089. [Google Scholar] [CrossRef] [PubMed]
- Lale, A.; Öz, B.; Akcan, A.C.; Sözüer, E.M.; Arıkan, T.B.; Gök, M. Determination of risk factors causing hypocalcaemia after thyroid surgery. Asian J. Surg. 2019, 42, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.E.; Unlu, M.T.; Aygun, N.; Kostek, M.; Tufan, A.E.; Yanar, C.; Yuksel, A.; Baran, E.; Cakir, Y.; Uludag, M. The Reality of Hypoparathyroidism After Thyroidectomy: Which Risk Factors are Effective? A Single-Center Study. Med. Bull. Sisli. Etfal. Hosp. 2022, 56, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Q.; Du, J.; Wang, Y.; Han, R.; Xu, C.; Chen, X.; Shu, M. Risk factors for postoperative hypocalcaemia after thyroidectomy: A systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 300060521996911. [Google Scholar] [CrossRef]
- Dağlar, G.; Kiliç, M.Ö.; Çelik, C.; Yüksel, C.; Terzioğlu, S.G.; Özden, S.; İçen, D. Is there a relationship between Vitamin D status and hypocalcemia after total thyroidectomy? Acta Endocrinol. 2016, 12, 291–296. [Google Scholar] [CrossRef]
- Singh, G.; Irshaidat, F.; Lau, C.; Pedoeem, A.; Feng, C.; Fariduddin, M.M.; Min, L.L.; Bansal, N. Advancing the Understanding of Vitamin D Status in Post-Thyroidectomy Hypocalcemia. Int. J. Endocrinol. 2021, 2021, 5598319. [Google Scholar] [CrossRef]
- Bove, A.; Dei Rocini, C.; Di Renzo, R.M.; Farrukh, M.; Palone, G.; Chiarini, S.; Staniscia, T. Vitamin D Deficiency as a Predictive Factor of Transient Hypocalcemia after Total Thyroidectomy. Int. J. Endocrinol. 2020, 2020, 8875257. [Google Scholar] [CrossRef]
- Spiliotis, J.; Vaxevanidou, A.; Sergouniotis, F.; Tsiveriotis, K.; Datsis, A.; Rogdakis, A.; Kekelos, S. Risk factors and consequences of incidental parathyroidectomy during thyroidectomy. Am. Surg. 2010, 76, 436–441. [Google Scholar] [CrossRef]
- Lin, Y.S.; Hsueh, C.; Wu, H.Y.; Yu, M.C.; Chao, T.C. Incidental parathyroidectomy during thyroidectomy increases the risk of postoperative hypocalcemia. Laryngoscope 2017, 127, 2194–2200. [Google Scholar] [CrossRef]
- Sitges-Serra, A.; Gallego-Otaegui, L.; Suárez, S.; Lorente-Poch, L.; Munné, A.; Sancho, J.J. Inadvertent parathyroidectomy during total thyroidectomy and central neck dissection for papillary thyroid carcinoma. Surgery 2017, 161, 712–719. [Google Scholar] [CrossRef]
- Toniato, A.; Boschin, I.M.; Piotto, A.; Pelizzo, M.; Sartori, P. Thyroidectomy and parathyroid hormone: Tracing hypocalcemia-prone patients. Am. J. Surg. 2008, 196, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Rosko, A.J.; Gay, B.L.; Reyes-Gastelum, D.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Surgeons’ Attitudes on Total Thyroidectomy vs Lobectomy for Management of Papillary Thyroid Microcarcinoma. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 667–669. [Google Scholar] [CrossRef]
- Song, J.; Qiu, W.; Yan, T.; Wu, B.; Guo, M.; Fan, Y.; Yang, Z. Comparison of Lobectomy and Total Thyroidectomy in Unilateral Papillary Thyroid Microcarcinoma Patients with Ipsilateral Lateral Lymph Node Metastasis without Gross Extrathyroidal Extension. World J. Surg. 2020, 44, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; De Leo, S.; Di Stefano, M.; Trevisan, M.; Moneta, C.; Vicentini, L.; Fugazzola, L. Total Thyroidectomy Versus Lobectomy for Thyroid Cancer: Single-Center Data and Literature Review. Ann. Surg. Oncol. 2021, 28, 4334–4344. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, R.; Sommerey, S.; Arabi, N.A.; Hallfeldt, K.K.J.; Stepp, H.; Gallwas, J.K.S. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 2017, 31, 3140–3145. [Google Scholar] [CrossRef]
- Tummers, Q.R.; Schepers, A.; Hamming, J.F.; Kievit, J.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose Methylene Blue. Surgery 2015, 158, 1323–1330. [Google Scholar] [CrossRef]
- Sound, S.; Okoh, A.; Yigitbas, H.; Yazici, P.; Berber, E. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg. Innov. 2019, 26, 774–779. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Sanders, M.E.; Broome, J.T.; Solórzano, C.C.; Mahadevan-Jansen, A. Establishing the Clinical Utility of Autofluorescence Spectroscopy for Parathyroid Detection. Surgery 2016, 159, 193–202. [Google Scholar] [CrossRef]
- De Leeuw, F.; Breuskin, I.; Abbaci, M.; Casiraghi, O.; Mirghani, H.; Ben Lakhdar, A.; Laplace-Builhé, C.; Hartl, D. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J. Surg. 2016, 40, 2131–2138. [Google Scholar] [CrossRef]
- Pata, G.; Casella, C.; Mittempergher, F.; Cirillo, L.; Salerni, B. Loupe magnification reduces postoperative hypocalcemia after total thyroidectomy. Am. Surg. 2010, 76, 1345–1350. [Google Scholar] [CrossRef]
- Cocchiara, G.; Cajozzo, M.; Fazzotta, S.; Palumbo, V.; Maione, C.; Buscemi, S.; Romano, G.; Fatica, F.; Spinelli, G.; Ficarella, S.; et al. Analisi dei fattori di rischio dell’ipoparatiroidismo transitorio e definitivo nei pazienti sottoposti a tiroidectomia. Clin. Ter. 2017, 168, 271–277. [Google Scholar] [CrossRef]
- Anagnostis, P.; Pliakos, I.; Panidis, S.; Chorti, A.; Stelmach, V.; Michalopoulos, A.; Papavramidis, T.S. Should total thyroidectomies be performed by high-volume endocrine surgeons? A cost-effectiveness analysis. Endocrine 2020, 67, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Wang, B.; Gong, Y.; Gong, R.; Li, Z.; Zhu, J. Risk factors of hypoparathyroidism following total thyroidectomy with central lymph node dissection. Medicine 2017, 96, e8162. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, M.A.; Lo Bianco, S.; Picardo, M.C.; Provenzano, D.; Buffone, A. How to avoid and to manage post-operative complications in thyroid surgery. Updates Surg. 2017, 69, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Chahardahmasumi, E.; Salehidoost, R.; Amini, M.; Aminorroaya, A.; Rezvanian, H.; Kachooei, A.; Iraj, B.; Nazem, M.; Kolahdoozan, M. Assessment of the Early and Late Complication after Thyroidectomy. Adv. Biomed. Res. 2019, 8, 14. [Google Scholar] [CrossRef]
- Dedivitis, R.A.; Aires, F.T.; Cernea, C.R. Hypoparathyroidism after thyroidectomy: Prevention, assessment and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 142–146. [Google Scholar] [CrossRef]
- Rosato, L.; Avenia, N.; Bernante, P.; De Palma, M.; Gulino, G.; Nasi, P.G.; Pelizzo, M.R.; Pezzullo, L. Complications of thyroid surgery: Analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J. Surg. 2004, 28, 271–276. [Google Scholar] [CrossRef]
- Abboud, B.; Sargi, Z.; Akkam, M.; Sleilaty, F. Risk factors for postthyroidectomy hypocalcemia. J. Am. Coll. Surg. 2002, 195, 456–461. [Google Scholar] [CrossRef]
- Del Río, L.; Castro, A.; Bernáldez, R.; Del Palacio, A.; Giráldez, C.V.; Lecumberri, B.; Alvarez-Escolá, C.; Fernández-Martínez, A. Parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia. Acta Otorrinolaringol. Esp. 2011, 62, 265–273. [Google Scholar] [CrossRef]
- Manzini, G.; Malhofer, F.; Weber, T. Can preoperative vitamin D deficiency predict postoperative hypoparathyroidism following thyroid surgery? Langenbeck’s Arch. Surg. 2019, 404, 55–61. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.P.; Murphy, M.S.; Sheahan, P. Vitamin D and risk of postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Kirkby-Bott, J.; Markogiannakis, H.; Skandarajah, A.; Cowan, M.; Fleming, B.; Palazzo, F. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J. Surg. 2011, 35, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Ku, Y.H.; Kim, H.I.; Lee, M.C.; Kim, M.J. Vitamin D level is not a predictor of hypocalcemia after total thyroidectomy. Langenbeck’s Arch. Surg. 2015, 400, 617–622. [Google Scholar] [CrossRef]
- Erbil, Y.; Barbaros, U.; Temel, B.; Turkoglu, U.; Işsever, H.; Bozbora, A.; Ozarmağan, S.; Tezelman, S. The impact of age, vitamin D3 level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am. J. Surg. 2009, 197, 439–446. [Google Scholar] [CrossRef]
- Erbil, Y.; Bozbora, A.; Ozbey, N.; Issever, H.; Aral, F.; Ozarmagan, S.; Tezelman, S. Predictive value of age and serum parathormone and vitamin d3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiter. Arch. Surg. 2007, 142, 1182–1187. [Google Scholar] [CrossRef]
- Cherian, A.J.; Ponraj, S.; Gowri, S.M.; Ramakant, P.; Paul, T.V.; Abraham, D.T.; Paul, M.J. The role of vitamin D in post-thyroidectomy hypocalcemia: Still an enigma. Surgery 2016, 159, 532–538. [Google Scholar] [CrossRef]
- Lin, Y.; Ross, H.L.; Raeburn, C.D.; DeWitt, P.E.; Albuja-Cruz, M.; Jones, E.L.; McIntyre, R.C., Jr. Vitamin D deficiency does not increase the rate of postoperative hypocalcemia after thyroidectomy. Am. J. Surg. 2012, 204, 888–894. [Google Scholar] [CrossRef]
- Chia, S.H.; Weisman, R.A.; Tieu, D.; Kelly, C.; Dillmann, W.H.; Orloff, L.A. Prospective study of perioperative factors predicting hypocalcemia after thyroid and parathyroid surgery. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 41–45. [Google Scholar] [CrossRef]

| Clinical Characteristics of Patients | n (%) |
|---|---|
| Number of patients | 211 (100%) |
| Number of RLNs at risk of damage, n (%) | 371 (100%) |
| Age, mean ± standard deviation, median years Minimum/maximum age, years | 51.7 ± 14.54; 52 16/82 |
| Gender (Female: Male) | 165:46 (3.6:1) |
| BMI, mean ± standard deviation, median kg/m2 BMI minimum/maximum | 28.07 ± 5.7; 27.5 16.26/44.96 |
| Thyroid volume (V), mean ± standard deviation, median mL V minimum/maximum, mL | 44.35 ± 57.3; 29.6 3.6/650 |
| |
| 39 (18.5%) |
| 157 (74.5%) |
| 15 (7%) |
| 67 (31.8%) |
| 51 (24.2%) |
| |
| 150 (71.1%) |
| 14 (6.65%) |
| 18 (8.5%) |
| 29 (13.75%) |
| 201 (95.3%) |
| 10 (4.7%) |
| |
| 156 (73.93%) |
| 2 (0.95%) |
| 2 (0.95%) |
| 51 (24.17%) |
| Operation time, mean ± standard deviation, median min Shortest/longest time | 97.87 ± 31.13, 95 30/185 |
| Surgeon’s experience, n (%) | |
| 156 (73.93%) |
| 55 (26.07%) |
| Number of Patients with Bilateral Thyroid Resection, n (%) | 160 | (100%) | 100% |
| Postoperative hypoparathyroidism in the immediate post-thyroid surgery period (PTH < 15 pg/mL), n (%) | 25 | 15.63% | |
| 19 | 76% | 11.88% |
| 23 | 92% | 14.38% |
| 9 | 36% | 5.63% |
| 16 | 64% | 10% |
| Postoperative permanent hypoparathyroidism | 3 | (100%) | 1.875% |
| Postoperative hypocalcemia (Ca < 8.8 mg/dL), n (%) | 72 | 100% | 45% |
| 21 | 29.17% | 13.13% |
| 23 | 31.94% | 14.38% |
| 19 | 26.39% | 11.88% |
| 43 | 55.72% | 26.88% |
| Postoperative hypocalcemia persistent | 5 | 100% | 3.125% |
| Risk Factors for Complications | Number of Patients n = 160 (100%) | Hypoparathyroidism PTH < 15 pg/mL | Hypocalcemia Ca < 8.8 mg/dL | |||
|---|---|---|---|---|---|---|
| Number of Patients at Risk n (100%) | Number of Patients n = 25 (100%) | Number of Patients at Risk n (100%) | Number of Patients n = 72 (100%) | |||
| Age | <65 years | 121 (75.63%) | 17 (14.05%) | 68% | 55 (45.45%) | 76.39% |
| ≥65 years | 39 (24.37%) | 8 (20.51%) | 32% | 17 (43.59%) | 23.61% | |
| Gender | Women | 129 (80.63%) | 20 (15.5%) | 80% | 61 (47.29%) | 84.72% |
| Men | 31 (19.37%) | 5 (16.13%) | 20% | 11 (35.48%) | 15.28% | |
| BMI (kg/m2) | Underweight (≤18.5) | 2 (1.25%) | 1 (50%) | 4% | 2 (100%) | 2.78% |
| Normal body weight (18.5–24.9) | 50 (31.25%) | 10 (20%) | 40% | 26 (52%) | 36.11% | |
| Overweight (25–29.9) | 62 (38.75%) | 9 (14.52%) | 36% | 28 (45.16%) | 38.89% | |
| Obesity (>30) | 46 (28.75%) | 5 (10.87%) | 20% | 16 (34.78%) | 22.22% | |
| Clinical diagnosis | Nodular goiter | 106 (66.25%) | 18 (16.98%) | 72% | 47 (44.34%) | 65.28% |
| Toxic nodular goiter | 14 (8.75%) | 1 (7.14%) | 4% | 9 (64.29%) | 12.5% | |
| Graves- Basedow disease | 18 (11.25%) | 2 (11.11%) | 8% | 9 (50.00%) | 12.5% | |
| Thyroid cancer | 22 (13.75%) | 4 (18.18%) | 16% | 7 (31.82%) | 9.72% | |
| Focal changes in the thyroid gland | Single | 16 (10%) | 3 (18.75%) | 12% | 7 (43.75%) | 9.72% |
| Plural | 129 (80.63%) | 21 (16.28%) | 84% | 58 (44.96%) | 80.56% | |
| Parenchymal goiter | 15 (9.37%) | 1 (6.67%) | 4% | 7 (46.67%) | 9.72% | |
| Total volume | ≤25 mL | 47 (29.3%) | 6 (12.77%) | 24% | 24 (51.06%) | 33.33% |
| 25–50 mL | 73 (45.63%) | 13 (17.81%) | 52% | 25 (34.25%) | 34.73% | |
| >50 mL | 40 (25.00%) | 6 (15.0%) | 24% | 23 (57.5%) | 31.94% | |
| Displaced/constricted trachea | 42 (26.25%) | 5 (11.90%) | 20% | 22 (52.38%) | 30.55% | |
| Retrosternal goiter | 59 (36.87%) | 9 (15.25%) | 36% | 30 (50.85%) | 41.67% | |
| Autoimmune disease | 25 (15.62%) | 3 (12.0%) | 12% | 14 (56.0%) | 19.4% | |
| Operation | Primary | 155 (96.88%) | 23 (14.84%) | 92% | 69 (44.52%) | 95.83% |
| Secondary | 5 (3.12%) | 2 (40.0%) | 8% | 3 (60.0%) | 4.17% | |
| Scope of thyroid surgery | Total | 156 (97.5%) | 25 (16.03%) | 100% | 71 (45.51%) | 98.61% |
| Partial | 4 (2.5%) | 0 (0%) | 0% | 1 (25.0%) | 1.39% | |
| Experienced surgeon | ≤50 operation/year | 40 (25.0%) | 11 (27.5%) | 44% | 22 (55.0%) | 30.55% |
| >50 operation/year | 120 (75.0%) | 14 (11.67%) | 56% | 50 (41.67%) | 69.55% | |
| Vitamin D3 levels before surgery, ng/mL | Deficiency [≤30]. | 84 (52.5%) | 16 (19.05%) | 64% | 47 (55.95%) | 65.28% |
| Normal level [>30]. | 76 (47.5%) | 9 (11.84%) | 36% | 25 (32.89%) | 34.72% | |
| Risk Factors for Complications | Hypoparathyroidism PTH < 15 pg/mL | ||||
|---|---|---|---|---|---|
| p | Odds Quotient OR | OR − 95% CI | OR + 95% CI | ||
| Age of patients | word free | 0.100 | 0.27 | 0.05 | 1.28 |
| regression coefficient | 0.617 | 0.99 | 0.96 | 1.02 | |
| BMI | word free | 0.685 | 0.62 | 0.06 | 5.95 |
| regression coefficient | 0.286 | 0.95 | 0.88 | 1.03 | |
| Thyroid volume | word free | 0.000 | 0.18 | 0.10 | 0.31 |
| regression coefficient | 0.986 | 1.00 | 0.99 | 1.00 | |
| Duration of operations | word free | 0.008 | 0.11 | 0.02 | 0.57 |
| regression coefficient | 0.549 | 1.00 | 0.99 | 1.01 | |
| PTH levels prior to surgery | word free | 0.366 | 0.58 | 0.17 | 1.88 |
| regression coefficient | 0.055 | 0.98 | 0.96 | 1.00 | |
| Calcium levels before surgery | word free | 0.252 | 0.00 | 0.00 | 28.66 |
| regression coefficient | 0.460 | 1.36 | 0.59 | 3.12 | |
| Phosphorus levels prior to surgery | word free | 0.273 | 0.25 | 0.02 | 2.94 |
| regression coefficient | 0.796 | 0.91 | 0.46 | 1.79 | |
| Vitamin D3 levels before surgery | word free | 0.023 | 0.25 | 0.07 | 0.83 |
| regression coefficient | 0.606 | 0.99 | 0.95 | 1.02 | |
| Risk Factors for Complications | Number of Patients n (100%) | Level of Significance p | Odds Ratio OR | |
|---|---|---|---|---|
| Number of Patients n (%) With Hypoparathyroidism: PTH < 15 pg/mL | ||||
| Gender | Women | Men | ||
| 129 (100%) | 31 (100%) | |||
| 20 (15.5%) | 5 (16.13%) | 0.931 | 2.77 | |
| Operation | Primary | Secondary | ||
| 155 (100%) | 5 (100%) | |||
| 23 (14.84%) | 2 (40.00%) | 0.127 | 24.16 | |
| Clinical diagnosis | Nodular goitre/ Toxic nodular goiter | Graves-Basedow’s disease/Thyroid cancer | ||
| 106 (100%) 14 (100%) | 18 (100%) 22 (100%) | |||
| 18 (16.98%) 1 (7.14%) | 2 (11.11%) 4 (18.18%) | 0.729 | - | |
| Autoimmune disease | YES | NO | ||
| 25 (100%) | 135 (100%) | |||
| 3 (12.0%) | 22 (16.30%) | 0.586 | 2.54 | |
| Type of focal lesions in thyroid ultrasound | Single tumor | Multiple nodules/ Parenchymal goiter | ||
| 16 (100%) | 129 (100%) 15 (100%) | |||
| 3 (18.75%) | 21 (16.28%) 1 (6.67%) | 0.584 | - | |
| Displaced trachea/ Constricted (X-ray) | YES | NO | ||
| 42 (100%) | 118 (100%) | |||
| 5 (11.90%) | 20 (16.95%) | 0.439 | 1.89 | |
| Retrosternal goiter | YES | NO | ||
| 59 (100%) | 101 (5.71%) | |||
| 9 (15.25%) | 16 (15.84%) | 0.921 | 2.32 | |
| Experienced surgeon | >50 operation/year | ≤50 operation/year | ||
| 120 (100%) | 40 (100%) | |||
| 114 (11.67%) | 11 (27.5%) | 0.016 | 6.99 | |
| Total operation | YES | NO | ||
| 156 (100%) | 4 (100%) | |||
| 25 (16.03%) | 0 (0%) | 0.383 | - | |
| Vitamin D deficiency | YES | NO | ||
| 84 (100%) | 76 (100%) | |||
| 16 (19.05%) | 9 (11.84%) | 0.210 | 4.23 | |
| Risk Factors for Complications | Postoperative Hypocalcemia (Ca < 8.8 mg/dL) | ||||
|---|---|---|---|---|---|
| p | Odds Quotient OR | OR − 95% CI | OR + 95% CI | ||
| Age of patients | word free | 0.312 | 1.82 | 0.56 | 5.90 |
| regression coefficient | 0.163 | 0.98 | 0.96 | 1.00 | |
| BMI | word free | 0.278 | 2.45 | 0.48 | 12.46 |
| regression coefficient | 0.177 | 0.96 | 0.90 | 1.01 | |
| Thyroid volume | word free | 0.041 | 0.62 | 0.39 | 0.98 |
| regression coefficient | 0.125 | 1.00 | 0.99 | 1.01 | |
| Duration of operations | word free | 0.050 | 0.30 | 0.09 | 1.00 |
| regression coefficient | 0.090 | 1.00 | 0.99 | 1.02 | |
| PTH levels prior to surgery | word free | 0.771 | 1.13 | 0.49 | 2.60 |
| regression coefficient | 0.412 | 0.99 | 0.98 | 1.00 | |
| Calcium levels before surgery | word free | 0.061 | 3.21 | 0.76 | 1.35 |
| regression coefficient | 0.051 | 0.53 | 0.28 | 1.00 | |
| Phosphorus levels prior to surgery | word free | 0.237 | 0.34 | 0.05 | 2.02 |
| regression coefficient | 0.330 | 1.27 | 0.78 | 2.07 | |
| Vitamin D3 levels before surgery | word free | 0.298 | 1.60 | 0.65 | 3.94 |
| regression coefficient | 0.117 | 0.97 | 0.95 | 1.00 | |
| Risk Factors for Complications | Number of Patients n (100%) | Significance Level p | Odds Ratio OR | |
|---|---|---|---|---|
| Number of Patients n (%) With Hypocalcemia (Ca < 8.8 mg/dL) | ||||
| Gender | Women | Men | ||
| 129 (100%) | 31 (100%) | |||
| 61 (47.29%) | 11 (35.48%) | 0.235 | 3.67 | |
| Operation | Primary | Secondary | ||
| 155 (100%) | 5 (100%) | |||
| 69 (44.52%) | 3 (60.00%) | 0.493 | 11.50 | |
| Clinical diagnosis | Nodular goitre/ Toxic nodular goiter | Graves-Basedow disease Thyroid cancer | ||
| 106 (100%) 14 (100%) | 18 (100%) 22 (100%) | |||
| 47 (44.34%) 9 (64.29%) | 9 (50.00%) 7 (31.82%) | 0.278 | - | |
| Autoimmune disease | YES | NO | ||
| 25 (100%) | 135 (100%) | |||
| 14 (56.0%) | 58 (42.96%) | 0.228 | 3.99 | |
| Focal changes in the thyroid gland | Single tumor | Multiple nodules/ Parenchymal goitre | ||
| 16 (100%) | 129 (100%) 15 (100%) | |||
| 7 (43.75%) | 58 (44.96%) 7 (46.67%) | 0.986 | - | |
| Displaced trachea (X-ray) | YES | NO | ||
| 42 (100%) | 118 (100%) | |||
| 22 (52.38%) | 50 (42.37%) | 0.262 | 3.03 | |
| Retrosternal goiter | YES | NO | ||
| 59 (100%) | 101 (100%) | |||
| 30 (50.85%) | 42 (41.58%) | 0.255 | 2.77 | |
| Experienced surgeon | >50 operation/year | ≤50 operation/year | ||
| 120 (100%) | 40 (100%) | |||
| 50 (41.67%) | 22 (55.0%%) | 0.142 | 3.51 | |
| Total operation | YES | NO | ||
| 156 (100%) | 4 (100%) | |||
| 71 (45.51%) | 1 (25%) | 0.415 | 3.92 | |
| Vitamin D deficiency | YES | NO | ||
| 84 (100%) | 76 (100%) | |||
| 44 (52.38%) | 28 (36.84%) | 0.048 | 3.55 | |
| Hypoparathyroidism | YES | NO | ||
| 25 (100%) | 135 (100%) | |||
| 23 (92.00%) | 49 (30.63%) | p < 0.0001 | 89.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sępek, M.; Marciniak, D.; Głód, M.; Kaliszewski, K.; Rudnicki, J.; Wojtczak, B. Risk Factors for Calcium-Phosphate Disorders after Thyroid Surgery. Biomedicines 2023, 11, 2299. https://doi.org/10.3390/biomedicines11082299
Sępek M, Marciniak D, Głód M, Kaliszewski K, Rudnicki J, Wojtczak B. Risk Factors for Calcium-Phosphate Disorders after Thyroid Surgery. Biomedicines. 2023; 11(8):2299. https://doi.org/10.3390/biomedicines11082299
Chicago/Turabian StyleSępek, Monika, Dominik Marciniak, Mateusz Głód, Krzysztof Kaliszewski, Jerzy Rudnicki, and Beata Wojtczak. 2023. "Risk Factors for Calcium-Phosphate Disorders after Thyroid Surgery" Biomedicines 11, no. 8: 2299. https://doi.org/10.3390/biomedicines11082299