Effects of the Co-Overexpression of the BCL and BDNF Genes on the Gamma-Aminobutyric Acid-Ergic Differentiation of Wharton’s-Jelly-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolating, Culturing and Characterizing the WJ-MSC
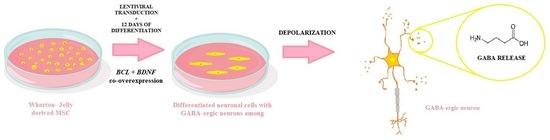
2.2. Lentiviral Transduction and Neuronal Differentiation
2.3. RT-qPCR
2.4. Evaluating the GAD67 Protein Expression
2.5. Gamma-Aminobutyric Acid Release Analysis
2.6. Statistical Analysis
3. Results
3.1. The Co-Overexpression of BCL and BDNF Affects the Expression Level of the Neuronal Pathway Genes
3.2. The Co-Overexpression of BCL and BDNF Affects the GAD67 Protein Expression Level
3.3. The Co-Overexpression of BCL and BDNF Affects the Release of Gamma-Aminobutyric Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 5, 892–905. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, Y.; Lee, S.; Kim, K.; Song, M.; Lee, J. Mesenchymal Stem Cell Therapy and Alzheimer’s Disease: Current Status and Future Perspectives. J. Alzheimers Dis. 2020, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Heris, R.M.; Shirvaliloo, M.; Abbaspour-Aghdam, S.; Hazrati, A.; Shariati, A.; Youshanlouei, H.R.; Niaragh, F.J.; Valizadeh, H.; Ahmadi, M. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem. Cell Res. Ther. 2022, 1, 371. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem. Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef]
- Yasuhara, T.; Matsukawa, N.; Hara, K.; Maki, M.; Ali, M.M.; Yu, S.J.; Bae, E.; Yu, G.; Xu, L.; McGrogan, M.; et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem. Cells Dev. 2009, 18, 1501–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.H.; Li, Y.; Chen, J.; Zacharek, A.; Gao, Q.; Kapke, A.; Lu, M.; Raginski, K.; Vanguri, P.; Smith, A.; et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.R.; Duan, W.M.; Reyes, M.; Keene, C.D.; Verfaillie, C.M.; Low, W.C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 2002, 174, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.H.; Ye, M.; Ding, X.S.; Han, Q.; Zhang, C.; Liu, X.F.; Huang, H.; Wu, E.B.; Huang, H.F.; Gu, X.S. Protective effects of MCI-186 on transplantation of bone marrow stromal cells in rat ischemic stroke model. Neuroscience 2012, 223, 315–324. [Google Scholar] [CrossRef]
- Li, Y.; Chopp, M.; Chen, J.; Wang, L.; Gautam, S.C.; Xu, Y.X.; Zhang, Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow Metab. 2000, 20, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Shichinohe, H.; Kuroda, S.; Lee, J.B.; Nishimura, G.; Yano, S.; Seki, T.; Ikeda, J.; Tamura, M.; Iwasaki, Y. In vivo tracking of bone marrow stromal cells transplanted into mice cerebral infarct by fluorescence optical imaging. Brain Res. Brain Res. Protoc. 2004, 13, 166–175. [Google Scholar] [CrossRef]
- Borkowska, P.; Zielinska, A.; Paul-Samojedny, M.; Stojko, R.; Kowalski, J. Synergistic Effect of the Long-Term Overexpression of Bcl-2 and BDNF Lentiviral in Cell Protecting against Death and Generating TH Positive and CHAT Positive Cells from MSC. Int. J. Mol. Sci. 2021, 13, 7086. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, P.; Morys, J.; Zielinska, A.; Sadlocha, M.; Kowalski, J. Survival and Neurogenesis-Promoting Effects of the Co-Overexpression of BCLXL and BDNF Genes on Wharton’s Jelly-Derived Mesenchymal Stem Cells. Life 2022, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Hayase, M.; Kitada, M.; Wakao, S.; Itokazu, Y.; Nozaki, K.; Hashimoto, N.; Takagi, Y.; Dezawa, M. Committed Neural Progenitor Cells Derived from Genetically Modified Bone Marrow Stromal Cells Ameliorate Deficits in a Rat Model of Stroke. J. Cereb. Blood Flow Metab. 2009, 8, 1409–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkowska, P.; Zielińska, A.; Paul-Samojedny, M.; Stojko, R.; Kowalski, J. Evaluation of reference genes for quantitative real-time PCR in Wharton’s Jelly-derived mesenchymal stem cells after lentiviral transduction and differentiation. Mol. Biol. Rep. 2020, 47, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Bartsch, U.; Stocking, C.; Fehse, B. A multicolor panel of novel lentiviral “gene ontology” (LeGO) vectors for functional gene analysis. Mol. Ther. 2008, 16, 698–706. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; De Lima Barros, P.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.E.; Beal, M.F. Oxidative Damage in Huntington’s Disease Pathogenesis. Antioxid. Redox. Signal 2006, 8, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Godin, J.D.; Poizat, G.; Hickey, M.A.; Maschat, F. Mutant huntingtin-impaired degradation of β-catenin causes neurotoxicity in Huntington’s disease. EMBO J. 2010, 29, 2433–2445. [Google Scholar] [CrossRef] [Green Version]
- Navone, S.E.; Marfia, G.; Canzi, L.; Ciusani, E.; Canazza, A.; Visintini, S.; Campanella, R.; Parati, E.A. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J. Orthop. Res. 2012, 9, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- San José, I.; Vázquez, E.; García-Atares, N.; Huerta, J.J.; Vega, J.A.; Represa, J. Differential expression of microtubule associated protein MAP-2 in developing cochleovestibular neurons and its modulation by neurotrophin. Int. J. Dev. Biol. 1997, 3, 509–519. [Google Scholar]
- Valeri, A.; Chiricosta, L.; Gugliandolo, A.; Pollastro, F.; Salamone, S.; Zingale, V.D.; Silvestro, S.; Mazzon, E. Cannabinerol and NSC-34 Transcriptomic Analysis: Is the Dose Who Makes Neuronal Differentiation? Int. J. Mol. Sci. 2022, 7, 7541. [Google Scholar] [CrossRef] [PubMed]
- Romanczyk, T.B.; Weickert, C.S.; Webster, M.J.; Herman, M.M.; Akil, M.; Kleinman, E.J. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur. J. Neurosci. 2002, 2, 269–280. [Google Scholar] [CrossRef]
- Wenceslau, C.V.; de Souza, D.M.; Mambelli-Lisboa, N.C.; Ynoue, L.H.; Araldi, R.P.; da Silva, J.M.; Pagani, E.; Haddad, M.S.; Kerkis, I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington’s Disease 3-NP Rat Model. Cells 2022, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Chen, H.; Huang, W.; Xu, S.; Liu, P.; Zou, W.; Pang, M.; Xu, Y.; Bai, X.; Liu, B.; et al. hUC-MSC-mediated recovery of subacute spinal cord injury through enhancing the pivotal subunits β3 and γ2 of the GABAA receptor. Theranostics 2022, 7, 3057–3078. [Google Scholar] [CrossRef]
- Mauri, M.; Lentini, D.; Gravati, M.; Foudah, D.; Biella, G.; Costa, B.; Toselli, M.; Parenti, M.; Coco, S. Mesenchymal stem cells enhance GABAergic transmission in co-cultured hippocampal neurons. Mol. Cell Neurosci. 2012, 4, 395–405. [Google Scholar] [CrossRef]
- Bardoni, R.; Ghirri, A.; Salio, C.; Prandini, M.; Merighi, A. BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Dev. Neurobiol. 2007, 7, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Pezet, S.; Cunningam, J.; Patel, J.; Grist, J.; Gavazzi, I.; Lever, I.J.; Malcangio, M. BDNF modulates sensory neuronsynaptic activity by a facilitation of GABA transmissionin the dorsal horn. Mol. Cell Neurosci. 2002, 21, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Meyerrose, T.; Olson, S.; Pontow, S.; Kalomoiris, S.; Jung, Y.; Annett, G.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2010, 62, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, K.; Sen, D. Mesenchymal Stem Cells as a Source of Dopaminergic Neurons: A Potential Cell Based Therapy for Parkinson’s Disease. Curr. Stem. Cell Res. Ther. 2017, 12, 326–347. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, M.; Kianian, F.; Kadkhodaee, M.; Seifi, B.; Ashabi, G.; Akhondzadeh, F.; Adelipour, M.; Izad, M.; Abdolmohammadi, K. Mesenchymal stem cells and their conditioned medium as potential therapeutic strategies in managing comorbid anxiety in rat sepsis induced by cecal ligation and puncture. Iran J. Basic Med. Sci. 2022, 25, 690–697. [Google Scholar] [PubMed]
- Shwartz, A.; Betzer, O.; Kronfeld, N.; Kazimirsky, G.; Cazacu, S.; Finniss, S.; Lee, H.K.; Motiei, M.; Dagan, S.Y.; Popovtzer, R.; et al. Therapeutic Effect of Astroglia-like Mesenchymal Stem Cells Expressing Glutamate Transporter in a Genetic Rat Model of Depression. Theranostics 2017, 7, 2690–2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrutia, M.; Fernández, S.; González, M.; Vilches, R.; Rojas, P.; Vásquez, M.; Kurte, M.; Vega-Letter, A.M.; Carrión, F.; Figueroa, F.; et al. Overexpression of Glutamate Decarboxylase in Mesenchymal Stem Cells Enhances Their Immunosuppressive Properties and Increases GABA and Nitric Oxide Levels. PLoS ONE 2016, 11, e0163735. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkowska, P.; Morys, J.; Zielinska, A.; Kowalski, J. Effects of the Co-Overexpression of the BCL and BDNF Genes on the Gamma-Aminobutyric Acid-Ergic Differentiation of Wharton’s-Jelly-Derived Mesenchymal Stem Cells. Biomedicines 2023, 11, 1751. https://doi.org/10.3390/biomedicines11061751
Borkowska P, Morys J, Zielinska A, Kowalski J. Effects of the Co-Overexpression of the BCL and BDNF Genes on the Gamma-Aminobutyric Acid-Ergic Differentiation of Wharton’s-Jelly-Derived Mesenchymal Stem Cells. Biomedicines. 2023; 11(6):1751. https://doi.org/10.3390/biomedicines11061751
Chicago/Turabian StyleBorkowska, Paulina, Julia Morys, Aleksandra Zielinska, and Jan Kowalski. 2023. "Effects of the Co-Overexpression of the BCL and BDNF Genes on the Gamma-Aminobutyric Acid-Ergic Differentiation of Wharton’s-Jelly-Derived Mesenchymal Stem Cells" Biomedicines 11, no. 6: 1751. https://doi.org/10.3390/biomedicines11061751