Effect of Various Protocols of Pre-Emptive Pulpal Laser Analgesia on Enamel Surface Morphology Using Scanning Electron Microscopy: An Ex Vivo Study
Abstract
:1. Introduction
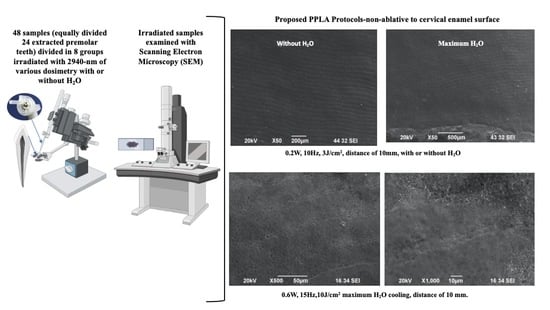
2. Materials and Methods
2.1. Study Design
2.2. Preparation of the Enamel Samples
2.3. Experimental Design
2.4. Laser Irradiation Process
2.5. Scanning Electron Microscopy (SEM)
2.5.1. SEM Description
2.5.2. SEM Preparation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, L.J. Technical Brief: Laser Analgesia with the ER: YAG Laser; University of Queensland: Brisbane, Australia, 1990; pp. 1–11. [Google Scholar]
- Neychev, D.; Chenchev, I.; Simitchiev, K. Analysis of postoperative pain after extraction of impacted mandibular third molars and administration of preemptive analgesia. J. IMAB 2017, 23, 1697–1701. [Google Scholar] [CrossRef] [Green Version]
- Walsh, L.J. Laser Analgesia with Pulsed Infrared Lasers: Theory and Practice. J. Oral Laser Appl. 2008, 8, 7–16. [Google Scholar]
- Chen, W. The Clinical applications for the Er, Cr: YSGG laser system. Chen Laser Inst. 2011, 12, 42–86. [Google Scholar]
- Fulop, A.M.; Dhimmer, S.; Deluca, J.R.; Johanson, D.D.; Lenz, R.V.; Patel, K.B.; Douris, P.C.; Enwemeka, C.S. A meta-analysis of the efficacy of laser phototherapy on pain relief. Clin. J. Pain 2010, 26, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Whitters, C.J.; Hall, A.; Creanor, S.L.; Moseley, H.; Gilmour, W.H.; Strang, R.; Saunders, W.P.; Orchardson, R. A clinical study of pulsed Nd: YAG laser-induced pulpal analgesia. J. Dent. 1995, 23, 145–150. [Google Scholar] [CrossRef]
- Chan, A.; Armati, P.; Moorthy, A.P.P. Pulsed Nd:YAG Laser Induces Pulpal Analgesia: A Randomized Clinical Trial. J. Dent. Res. 2012, 91, S79–S84. [Google Scholar] [CrossRef]
- Chan, A.; Punnia-Moorthy, A.; Armati, P. Low-power pulsed Nd: YAG laser irradiation for pre-emptive anaesthesia: A morphological and histological study. Laser Ther. 2014, 23, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; George, R.; Walsh, L.J. Pulpal response following photo-biomodulation with a 904-nm diode laser: A double-blind clinical study. Lasers Med. Sci. 2016, 31, 1811–1817. [Google Scholar] [CrossRef]
- Genovese, M.D.; Olivi, G. Laser in paediatric dentistry: Patient acceptance of hard and soft tissue therapy. Eur. J. Paediatr. Dent. 2008, 9, 13–17. [Google Scholar]
- Veneva, E.; Belcheva, A. Placebo-controlled subjective and objective evalautio of laser analgesia efficacy—A case report. J. IMAB 2019, 25, 2343–2348. [Google Scholar] [CrossRef]
- Angelieri, F.; Sousa, M.V.D.S.; Kanashiro, L.K.; Siquera, D.F.; Maltagliati, L.A. Effects of low intensity laser on pain sensitivity during orthodontic movement. Dent. Press J. Orthod. 2011, 16, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Deana, N.F.; Zaror, C.; Sandoval, P.; Alves, N. Effectiveness of Low-Level Laser Therapy in Reducing Orthodontic Pain: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2017, 2017, 8560652. [Google Scholar] [CrossRef]
- Kodaka, T. Originak article scenning. Electron microscopic observations of surface prismless enamle formed by. Minute crystals in some humna permanent teeth. Anat. Sci. Int. 2003, 78, 79–84. [Google Scholar] [CrossRef]
- Li, C.; Risnes, S. SEM observations of retzius lines and prism cross-striations in human dental enamel after different acid etching regimes. Arch. Oral Biol. 2004, 49, 45–52. [Google Scholar] [CrossRef]
- Kodaka, T.; Kuroiwa, M.; Higashi, S. Structural and Distribution Patterns of Surface ‘Prismless’ Enamel in Human Permanent Teeth. Caries Res. 1991, 25, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Risnes, S.; Saeed, M.; Sehic, A. Scannng Electron Microscopy (SEM) Methods fordental Enamel. Methods Mol. Biol. 2019, 1922, 293–308. [Google Scholar] [CrossRef]
- Fava, M.; Watanabe, I.S.; Fava-de-moraes, F.; da Costa, L.R. Prismless enamel in human non-erupted deciduous molar teeth: A scanning electron microscopic study. Rev. Odontol. Univ. São Paulo 1997, 11, 239–243. [Google Scholar] [CrossRef]
- Poli, R.; Parker, S. Achieving Dental Analgesia with the Erbium Chromium Yttrium Scandium Gallium Garnet Laser (2780 nm): A Protocol for Painless Conservative Treatment. Photomed. Laser Surg. 2015, 33, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Garboczi, E.J.; Bentz, D.P.; Stutzman, P.E.; Mason, T.O. Estimation of the degree of hydration of blended cement pastes by a scanning electron microscope point-counting procedure. Cem. Concr. Res. 2004, 34, 1787–1793. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Dunant, C.F.; Ben-Haha, M.; Scrivener, K.L. A new quantification method based on SEM-EDS to assess fly ash composition and study the reaction of its individual components in hydrating cement paste. Cem. Concr. Res. 2015, 73, 111–122. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [Green Version]
- Elicherla, S.; Sahithi, V.; Saikiran, K.; Nunna, M.; Challa, R.; Nuvvula, S. Local anesthesia in pediatric dentistry: A literature review on current alternative techniques and approaches. JSAAPD 2021, 4, 148–154. [Google Scholar] [CrossRef]








| Laser Parameters | Group A | Group B | Group C | Group D | Group E | Group F | Group G | Group I |
|---|---|---|---|---|---|---|---|---|
| Average power (W) | 0.2 | 0.2 | 0.6 | 0.6 | 0.75 | 0.75 | 1 | 1 |
| Pulse frequency (Hz) | 10 | 10 | 15 | 15 | 15 | 15 | 20 | 20 |
| Energy per pulse (mJ) | 20 | 20 | 40 | 40 | 50 | 50 | 50 | 50 |
| Fluence (J/cm2) | 3 | 3 | 10 | 10 | 12 | 12 | 17 | 17 |
| Water spray (%) | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| Tip-to-tissue distance | 10 mm | |||||||
| Speed of movement | 2 mm/s | |||||||
| Tip type and diameter | 1.3 × 6.3 mm sapphire tip | |||||||
| Irradiation time | 30 s/sample | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belcheva, A.; Veneva, E.; Hanna, R. Effect of Various Protocols of Pre-Emptive Pulpal Laser Analgesia on Enamel Surface Morphology Using Scanning Electron Microscopy: An Ex Vivo Study. Biomedicines 2023, 11, 1077. https://doi.org/10.3390/biomedicines11041077
Belcheva A, Veneva E, Hanna R. Effect of Various Protocols of Pre-Emptive Pulpal Laser Analgesia on Enamel Surface Morphology Using Scanning Electron Microscopy: An Ex Vivo Study. Biomedicines. 2023; 11(4):1077. https://doi.org/10.3390/biomedicines11041077
Chicago/Turabian StyleBelcheva, Ani, Elitsa Veneva, and Reem Hanna. 2023. "Effect of Various Protocols of Pre-Emptive Pulpal Laser Analgesia on Enamel Surface Morphology Using Scanning Electron Microscopy: An Ex Vivo Study" Biomedicines 11, no. 4: 1077. https://doi.org/10.3390/biomedicines11041077