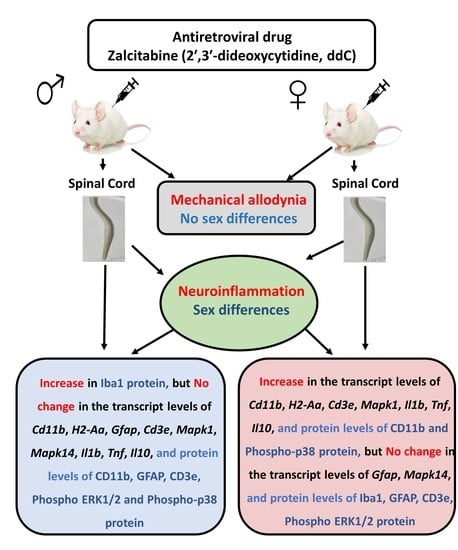
Sex Differences in the Expression of Neuroimmune Molecules in the Spinal Cord of a Mouse Model of Antiretroviral-Induced Neuropathic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Treatment
2.3. Assessment of Mechanical Allodynia
2.4. Animal Sacrifice and Tissue Isolation
2.5. RNA Extraction and cDNA Synthesis
2.6. Real Time-Polymerase Chain Reaction (RT-PCR)
- ΔCt = Ct target gene—Ct housekeeping gene. The housekeeping gene Ppia was used to normalize the number of transcripts of individual animal samples (ΔCt; n = 6 to 8 per group).
- ΔΔCt = ΔCt of experimental animals—Average of ΔCt of control animals.
- The fold change in the target genes = 2−ΔΔCT. These values were then used to calculate the mean ± standard error of the mean (SEM) or median and interquartile range of the relative expression of the target gene mRNA in the spinal cord of vehicle and ddC-treated mice.
2.7. WesTM Capillary-Based Protein Electrophoresis
2.8. Statistical Analysis
3. Results
3.1. Effects of ddC on Withdrawal Threshold to Mechanical Stimulation
3.2. Effect of ddC Treatment on the Gene and Protein Expression of Glial Cells and T Cells Markers in the Spinal Cord of Male and Female Mice
3.3. Effect of ddC Treatment on Gene and Protein Expression of Signaling Molecules ERK1/2 and p38 MAPK in the Spinal Cord of Male and Female Mice
3.4. Effect of ddC Treatment on Gene Expression of Pro-Inflammatory and Anti-Inflammatory Molecules (Il1b, Tnf and Il10) in the Spinal Cord of Male and Female Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croke, L. HIV Prevention and Treatment with ART: International Antiviral Society Updates Recommendations. Am. Fam. Physician 2019, 99, 395–396. [Google Scholar] [PubMed]
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/what-start-initial-combination-regimens-antiretroviral-naive (accessed on 21 September 2022).
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; Del Rio, C.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA 2020, 324, 1651–1669. [Google Scholar] [CrossRef]
- Badowski, M.; Perez, S.E.; Silva, D.; Lee, A. Two’s a Company, Three’s a Crowd: A Review of Initiating or Switching to a Two-Drug Antiretroviral Regimen in Treatment-Naive and Treatment-Experienced Patients Living with HIV-1. Infect. Dis. Ther. 2020, 9, 185–208. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Pinto, A.; Bhaskaran, K.; Dunn, D.; Weller, I.V. The risk of developing peripheral neuropathy induced by nucleoside reverse transcriptase inhibitors decreases over time: Evidence from the Delta trial. Antivir. Ther. 2008, 13, 289–295. [Google Scholar] [CrossRef]
- Dalakas, M.C. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001, 6, 14–20. [Google Scholar] [CrossRef]
- Kallianpur, A.R.; Hulgan, T. Pharmacogenetics of nucleoside reverse-transcriptase inhibitor-associated peripheral neuropathy. Pharmacogenomics 2009, 10, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, E.; Khajah, M.A.; Masocha, W. beta-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the Central Nervous System. Cell Mol. Neurobiol. 2018, 38, 53–71. [Google Scholar] [CrossRef]
- Joseph, E.K.; Chen, X.; Khasar, S.G.; Levine, J.D. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain 2004, 107, 147–158. [Google Scholar] [CrossRef]
- Munawar, N.; Oriowo, M.A.; Masocha, W. Antihyperalgesic Activities of Endocannabinoids in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Front. Pharm. 2017, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Levine, J.D. Mechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the rat. Mol. Pain 2007, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Paller, C.J.; Campbell, C.M.; Edwards, R.R.; Dobs, A.S. Sex-based differences in pain perception and treatment. Pain Med. 2009, 10, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [Green Version]
- Rosen, S.; Ham, B.; Mogil, J.S. Sex differences in neuroimmunity and pain. J. Neurosci. Res. 2017, 95, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef] [Green Version]
- VanRyzin, J.W.; Pickett, L.A.; McCarthy, M.M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev. Neurobiol. 2018, 78, 580–592. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Blomgren, K.; Harris, R.A. Uncovering sex differences of rodent microglia. J. Neuroinflammation 2021, 18, 74. [Google Scholar] [CrossRef]
- Green, T.; Flash, S.; Reiss, A.L. Sex differences in psychiatric disorders: What we can learn from sex chromosome aneuploidies. Neuropsychopharmacology 2019, 44, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Gold, S.M.; Willing, A.; Leypoldt, F.; Paul, F.; Friese, M.A. Sex differences in autoimmune disorders of the central nervous system. Semin. Immunopathol. 2019, 41, 177–188. [Google Scholar] [CrossRef]
- Zagni, E.; Simoni, L.; Colombo, D. Sex and Gender Differences in Central Nervous System-Related Disorders. Neurosci. J. 2016, 2016, 2827090. [Google Scholar] [CrossRef] [Green Version]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beggs, S.; Trang, T.; Salter, M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 2012, 15, 1068–1073. [Google Scholar] [CrossRef]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; DeLeo, J.A. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008, 38, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Costigan, M.; Moss, A.; Latremoliere, A.; Johnston, C.; Verma-Gandhu, M.; Herbert, T.A.; Barrett, L.; Brenner, G.J.; Vardeh, D.; Woolf, C.J.; et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 2009, 29, 14415–14422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Shi, Y.; Guo, K.; Tang, S.J. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Induce Pathological Pain through Wnt5a-Mediated Neuroinflammation in Aging Mice. J. Neuroimmune Pharm. 2018, 13, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Thomas, A. Indomethacin plus minocycline coadministration relieves chemotherapy and antiretroviral drug-induced neuropathic pain in a cannabinoid receptors-dependent manner. J. Pharm. Sci. 2019, 139, 325–332. [Google Scholar] [CrossRef]
- Sanna, M.D.; Quattrone, A.; Ghelardini, C.; Galeotti, N. PKC-mediated HuD-GAP43 pathway activation in a mouse model of antiretroviral painful neuropathy. Pharm. Res. 2014, 81, 44–53. [Google Scholar] [CrossRef]
- Wallace, V.C.; Blackbeard, J.; Segerdahl, A.R.; Hasnie, F.; Pheby, T.; McMahon, S.B.; Rice, A.S. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007, 130 Pt 10, 2688–2702. [Google Scholar] [CrossRef] [Green Version]
- Carey, L.M.; Xu, Z.; Rajic, G.; Makriyannis, A.; Romero, J.; Hillard, C.; Mackie, K.; Hohmann, A.G. Peripheral sensory neuron CB2 cannabinoid receptors are necessary for both CB2-mediated antinociceptive efficacy and sparing of morphine tolerance in a mouse model of anti-retroviral toxic neuropathy. Pharm. Res. 2023, 187, 106560. [Google Scholar] [CrossRef]
- Richner, M.; Jager, S.B.; Siupka, P.; Vaegter, C.B. Hydraulic Extrusion of the Spinal Cord and Isolation of Dorsal Root Ganglia in Rodents. J. Vis. Exp. 2017, e55226. [Google Scholar] [CrossRef] [Green Version]
- Masocha, W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J. NeuroImmunol. 2009, 214, 78–82. [Google Scholar] [CrossRef]
- Amin, D.N.; Vodnala, S.K.; Masocha, W.; Sun, B.; Kristensson, K.; Rottenberg, M.E. Distinct Toll-like receptor signals regulate cerebral parasite load and interferon alpha/beta and tumor necrosis factor alpha-dependent T-cell infiltration in the brains of Trypanosoma brucei-infected mice. J. Infect. Dis. 2012, 205, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Amin, D.N.; Kristensson, K.; Rottenberg, M.E. Differential invasion of Trypanosoma brucei brucei and lymphocytes into the brain of C57BL/6 and 129Sv/Ev mice. Scand. J. Immunol. 2008, 68, 484–491. [Google Scholar] [CrossRef]
- Masocha, W.; Rottenberg, M.E.; Kristensson, K. Minocycline impedes African trypanosome invasion of the brain in a murine model. Antimicrob. Agents ChemoTher. 2006, 50, 1798–1804. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.Y.; Masocha, W. Indomethacin augments lipopolysaccharide-induced expression of inflammatory molecules in the mouse brain. PeerJ 2020, 8, e10391. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Keogh, E. Sex Differences in Pain. Rev. Pain 2008, 2, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Kisucka, A.; Bimbova, K.; Bacova, M.; Galik, J.; Lukacova, N. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells 2021, 10, 1943. [Google Scholar] [CrossRef]
- Kuhn, J.A.; Vainchtein, I.D.; Braz, J.; Hamel, K.; Bernstein, M.; Craik, V.; Dahlgren, M.W.; Ortiz-Carpena, J.; Molofsky, A.B.; Molofsky, A.V.; et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. Elife 2021, 10. [Google Scholar]
- Nieto, F.R.; Clark, A.K.; Grist, J.; Hathway, G.J.; Chapman, V.; Malcangio, M. Neuron-immune mechanisms contribute to pain in early stages of arthritis. J. Neuroinflammation 2016, 13, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, A.C.; Smith, E.S.; Picker, M.J. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur. J. Pharm. 2002, 452, 163–173. [Google Scholar] [CrossRef]
- Parvathy, S.S.; Masocha, W. Coadministration of indomethacin and minocycline attenuates established paclitaxel-induced neuropathic thermal hyperalgesia: Involvement of cannabinoid CB1 receptors. Sci. Rep. 2015, 5, 10541. [Google Scholar] [CrossRef] [Green Version]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth 2013, 111, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; Strath, L.J. Sex differences in pain responses. Curr. Opin. Physiol. 2018, 6, 75–81. [Google Scholar] [CrossRef]
- Sorge, R.E.; Totsch, S.K. Sex Differences in Pain. J. Neurosci. Res. 2017, 95, 1271–1281. [Google Scholar] [CrossRef]
- Sanna, M.D.; Quattrone, A.; Mello, T.; Ghelardini, C.; Galeotti, N. The RNA-binding protein HuD promotes spinal GAP43 overexpression in antiretroviral-induced neuropathy. Exp. Neurol. 2014, 261, 343–353. [Google Scholar] [CrossRef]
- Sanna, M.D.; Peroni, D.; Quattrone, A.; Ghelardini, C.; Galeotti, N. Spinal RyR2 pathway regulated by the RNA-binding protein HuD induces pain hypersensitivity in antiretroviral neuropathy. Exp. Neurol. 2015, 267, 53–63. [Google Scholar] [CrossRef]
- Sanna, M.D.; Ghelardini, C.; Galeotti, N. Blockade of the spinal BDNF-activated JNK pathway prevents the development of antiretroviral-induced neuropathic pain. Neuropharmacology 2016, 105, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Ghelardini, C.; Galeotti, N. Spinal astrocytic c-Jun N-terminal kinase (JNK) activation as counteracting mechanism to the amitriptyline analgesic efficacy in painful peripheral neuropathies. Eur. J. Pharm. 2017, 798, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Naji-Esfahani, H.; Vaseghi, G.; Safaeian, L.; Pilehvarian, A.A.; Abed, A.; Rafieian-Kopaei, M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab. Anim. 2016, 50, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ahlstrom, F.H.G.; Matlik, K.; Viisanen, H.; Blomqvist, K.J.; Liu, X.; Lilius, T.O.; Sidorova, Y.; Kalso, E.A.; Rauhala, P.V. Spared Nerve Injury Causes Sexually Dimorphic Mechanical Allodynia and Differential Gene Expression in Spinal Cords and Dorsal Root Ganglia in Rats. Mol. Neurobiol. 2021, 58, 5396–5419. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.L.; Alley, J.F.; Wellman, L.; Beitz, A.J. Decreased spinal cord opioid receptor mRNA expression and antinociception in a Theiler’s murine encephalomyelitis virus model of multiple sclerosis. Brain Res. 2008, 1191, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Rahn, E.J.; Iannitti, T.; Donahue, R.R.; Taylor, B.K. Sex differences in a mouse model of multiple sclerosis: Neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol. Sex Differ. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackbeard, J.; Wallace, V.C.; O’Dea, K.P.; Hasnie, F.; Segerdahl, A.; Pheby, T.; Field, M.J.; Takata, M.; Rice, A.S. The correlation between pain-related behaviour and spinal microgliosis in four distinct models of peripheral neuropathy. Eur. J. Pain 2012, 16, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.Y.; Xiao, W.H.; Bennett, G.J. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011, 176, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Ouyang, H.; Liu, S.; Mata, M.; Fink, D.J.; Hao, S. TNFalpha is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behav. Immun 2011, 25, 1668–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Wang, G.; Zhang, F. Role of Peripheral Immune Cells-Mediated Inflammation on the Process of Neurodegenerative Diseases. Front. Immunol. 2020, 11, 582825. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatry 2016, 6, 283–293. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Lamkin, A.; Peterson, P.K.; Chao, C.C. Susceptibility to immunologically mediated fatigue in C57BL/6 versus Balb/c mice. Clin. Immunol. Immunopathol. 1996, 81, 161–167. [Google Scholar] [CrossRef]
- Koo, G.C.; Gan, Y.H. The innate interferon gamma response of BALB/c and C57BL/6 mice to in vitro Burkholderia pseudomallei infection. BMC Immunol. 2006, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, M.L.; Canzobre, M.C.; Pascuan, C.G.; Rios, H.; Wald, M.; Genaro, A.M. Stress induced cognitive deficit is differentially modulated in BALB/c and C57Bl/6 mice: Correlation with Th1/Th2 balance after stress exposure. J. NeuroImmunol. 2010, 218, 12–20. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Haiar, J.M.; Watt, M.J.; Newton, S.S. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress 2017, 20, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr. Drug Targets Inflamm. Allergy 2004, 3, 299–303. [Google Scholar] [CrossRef]
- Ji, R.R.; Gereau, R.W.T.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Bachstetter, A.D.; Van Eldik, L.J. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010, 1, 199–211. [Google Scholar]
- Berta, T.; Park, C.K.; Xu, Z.Z.; Xie, R.G.; Liu, T.; Lu, N.; Liu, Y.C.; Ji, R.R. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J. Clin. Investig. 2014, 124, 1173–1186. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.K.; D’Aquisto, F.; Gentry, C.; Marchand, F.; McMahon, S.B.; Malcangio, M. Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J. Neurochem. 2006, 99, 868–880. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [Green Version]















| Name | Polarity | Sequence 5′to 3′ | GenBank * |
|---|---|---|---|
| Ppia | Sense | GCTTTTCGCCGCTTGCT | X52803 |
| Anti-sense | CTCGTCATCGGCCGTGAT | ||
| Itgam | Sense | TGCTTACCTGGGTTATGCTTCTG | NM_008401 |
| Anti-sense | CCGAGGTGCTCCTAAAACCA | ||
| H2-Aa | Sense | CTGTGATCAACATCACATGGC | NM_010378 |
| Anti-sense | TTGTGGAAGGAATAGTCACGG | ||
| Gfap | Sense | ACAGCGGCCCTGAGAGAGAT | X02801 |
| Anti-sense | CTCCTCTGTCTCTTGCATGTTACTG | ||
| Cd3e | Sense | TGCTACACACCAGCCTCAAA | NM_007648 |
| Anti-sense | AGGTCCACCTCCACACAGTA | ||
| Mapk14 | Sense | AGGCCATGGTGCATGTGTGT | XR-004939533 |
| Anti-sense | AGTAGCTGGAGGAGGAGGAG | ||
| Mapk1 | Sense | GCTCACCCTTACCTGGAACA | NM_011952 |
| Anti-sense | GGACCAGATCCAAAAGGACA | ||
| Il1b | Sense | TGGTGTGTACGTTCCCATT | NM_008361 |
| Anti-sense | CAGCAGAGGCTTTTTTGTTG | ||
| Tnf | Sense | GGCTGCCCCGACTACGT | NM_013693 |
| Anti-sense | GACTTTCTCCTGGTATGAGATAGCAAA | ||
| Il10 | Sense | CAGCCGGGAAGACAATAACTG | NM_010548 |
| Anti-sense | CCGCAGCTCTAGGAGCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-HadlaQ, M.W.; Masocha, W. Sex Differences in the Expression of Neuroimmune Molecules in the Spinal Cord of a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Biomedicines 2023, 11, 875. https://doi.org/10.3390/biomedicines11030875
Al-HadlaQ MW, Masocha W. Sex Differences in the Expression of Neuroimmune Molecules in the Spinal Cord of a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Biomedicines. 2023; 11(3):875. https://doi.org/10.3390/biomedicines11030875
Chicago/Turabian StyleAl-HadlaQ, Maryam W., and Willias Masocha. 2023. "Sex Differences in the Expression of Neuroimmune Molecules in the Spinal Cord of a Mouse Model of Antiretroviral-Induced Neuropathic Pain" Biomedicines 11, no. 3: 875. https://doi.org/10.3390/biomedicines11030875