Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review
Abstract
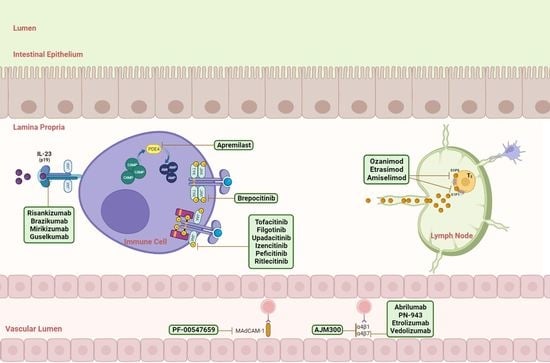
:1. Introduction
2. Targets for Small Molecules and Biologics
3. Biologics and Small Molecules
3.1. IL Inhibitors
3.1.1. Selective Inhibitors of IL-23
3.1.2. Risankizumab
3.1.3. Brazikumab
3.1.4. Guselkumab
3.1.5. Mirikizumab
3.2. TNF Inhibitors
3.2.1. AVX-470
3.2.2. OPRX-106
3.3. Anti-Adhesion Molecules
3.3.1. Etrolizumab
3.3.2. PN-943
3.3.3. PF-00547659
3.3.4. Abrilumab
3.3.5. Carotegrast Methyl (AJM300)
3.4. Sphingosine-1-Phosphate (S1P) Receptor Modulators
3.4.1. Fingolimod
3.4.2. Ozanimod
3.4.3. Etrasimod
3.4.4. Amiselimod
3.5. Phosphodiesterase 4 Inhibitors
Apremilast
3.6. Janus Kinase Inhibitors (JAKi)
3.6.1. Tofacitinib
3.6.2. Filgotinib
3.6.3. Upadacitinib
3.6.4. Peficitinib
3.6.5. Izencitinib (TD-1473)
3.7. TYK2 Inhibitors
3.7.1. Brepocitinib
3.7.2. Deucravacitinib
3.8. Toll-Like Receptor 9 (TLR9) Agonists
Cobitolimod
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nature Reviews. Gastroenterol. Hepatol. 2015, 13, 13–27. [Google Scholar]
- Klenske, E.; Bojarski, C.; Waldner, M.; Rath, T.; Neurath, M.F.; Atreya, R. Targeting mucosal healing in Crohn’s disease: What the clinician needs to know. Ther. Adv. Gastroenterol. 2019, 12, 1756284819856865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Sabino, J.; Verstockt, B.; Vermeire, S.; Ferrante, M. New biologics and small molecules in inflammatory bowel disease: An update. Ther. Adv. Gastroenterol. 2019, 12, 3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftychi, C.; Schwarzer, R.; Vlantis, K.; Wachsmuth, L.; Basic, M.; Wagle, P.; Neurath, M.F.; Becker, C.; Bleich, A.; Pasparakis, M.; et al. Temporally Distinct Functions of the Cytokines IL-12 and IL-23 Drive Chronic Colon Inflammation in Response to Intestinal Barrier Impairment. Immunity 2019, 51, 367–380.e4. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Assimakopoulos, S.F.; Konstantakis, C.; Triantos, C.C. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn’s disease. World J. Gastroenterol. 2018, 24, 4093. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.W.; Rennick Natalie JDavidson, D.M.; Hudak, S.A.; Lesley, R.E. IL-10-Deficient Mice Sustaining the Chronic Phase of Colitis in, Plays a Major Role in γ IL-12, But Not IFN. 2022. Available online: http://www.jimmunol.org/content/161/6/3143 (accessed on 7 January 2023).
- Mannon, P.J.; Fuss, I.J.; Mayer, L.; Elson, C.O.; Sandborn, W.J.; Present, D.; Dolin, B.; Goodman, N.; Groden, C.; Hornung, R.L.; et al. Anti–Interleukin-12 Antibody for Active Crohn’s Disease. N. Engl. J. Med. 2004, 351, 2069–2079. [Google Scholar] [CrossRef]
- Jefremow, A.; Neurath, M.F. All are Equal, Some are More Equal: Targeting IL 12 and 23 in IBD–A Clinical Perspective. Immunotargets Ther. 2020, 9, 289. [Google Scholar] [CrossRef]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Dolin, B.; Goodman, N.; Groden, C.; Hornung, R.L.; et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science 2006, 314, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 7422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2017, 53, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, M.; Salem, M.; Pedersen, J.; Nielsen, O.H. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013, 76, 1–8. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Uronis, J.M.; Jobin, C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. Am. J. Pathol. 2011, 179, 673–683. [Google Scholar] [CrossRef]
- Neubauer, H.; Cumano, A.; Müller, M.; Wu, H.; Huffstadt, U.; Pfeffer, K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998, 93, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Rodig, S.J.; Meraz, M.A.; White, J.M.; Lampe, P.A.; Riley, J.K.; Arthur, C.D.; King, K.L.; Sheehan, K.C.; Yin, L.; Pennica, D.; et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998, 93, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sphingosine-1 Phosphate Receptor Modulators: The Next Wave of Oral Therapies in Inflammatory Bowel Disease–Gastroenterology & Hepatology. Available online: https://www.gastroenterologyandhepatology.net/archives/may-2022/sphingosine-1-phosphate-receptor-modulators-the-next-wave-of-oral-therapies-in-inflammatory-bowel-disease/ (accessed on 9 January 2023).
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 2022, 399, 2015–2030. [Google Scholar] [CrossRef]
- Deepak, P.; Sandborn, W.J. Ustekinumab and Anti-Interleukin-23 Agents in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 603–626. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Panés, J.; Kaser, A.; Ferrante, M.; Louis, E.; Franchimont, D.; Dewit, O.; Seidler, U.; et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: A randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017, 389, 1699–1709. [Google Scholar] [CrossRef]
- Ferrante, M.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: Results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 2022, 399, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Panés, J.; Ferrante, M.; Kaser, A.; D’Haens, G.R.; Sandborn, W.J.; Louis, E.; Neurath, M.F.; Franchimont, D.; Dewit, O.; et al. Risankizumab in patients with moderate to severe Crohn’s disease: An open-label extension study. Lancet Gastroenterol. Hepatol. 2018, 3, 671–680. [Google Scholar] [CrossRef]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohns Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Long-Term Safety in the Open-Label Period of a Phase 2a Stud..: Official Journal of the American College of Gastroenterology|ACG. Available online: https://journals.lww.com/ajg/fulltext/2018/10001/long_term_safety_in_the_open_label_period_of_a.590.aspx (accessed on 7 October 2022).
- Janssen Announces, u.s. Fda Approval of Tremfyatm (Guselkumab) for the Treatment of Moderate to Severe Plaque Psoriasis|Johnson & Johnson. Available online: https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis (accessed on 11 January 2023).
- Cdjjlgaoaa, S. The efficacy and safety OF guselkumab induction therapy IN patients with moderately to severely active CROHN’S disease: Week 12 interim analyses from the phase 2 GALAXI 1 study. United Eur. Gastroenterol. J. 2020, 8, 64. [Google Scholar]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results From the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Feagan, B.G.; Hibi, T.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 537–549.e10. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Ferrante, M.; Bhandari, B.R.; Berliba, E.; Hibi, T.; D’Haens, G.R.; Tuttle, J.L.; Klekotka, P.; Friedrich, S.; Durante, M.; et al. Efficacy and Safety of Continued Treatment With Mirikizumab in a Phase 2 Trial of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2022, 20, 105–115.e14. [Google Scholar] [CrossRef]
- Harris, M.S.; Hartman, D.; Lemos, B.R.; Erlich, E.C.; Spence, S.; Kennedy, S.; Ptak, T.; Pruitt, R.; Vermeire, S.; Fox, B.S. AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J. Crohns Colitis 2016, 10, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Almon, E.; Shaaltiel, Y.; Sbeit, W.; Fich, A.; Schwartz, D.; Waterman, M.; Szlaifer, M.; Reuveni, H.; Amit-Cohen, B.C.; Alon, S.; et al. Novel Orally Administered Recombinant Anti-TNF Alpha Fusion Protein for the Treatment of Ulcerative Colitis: Results From a Phase 2a Clinical Trial. J. Clin. Gastroenterol. 2021, 55, 134. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; O’Byrne, S.; Keir, M.; Williams, M.; Lu, T.T.; Mansfield, J.C.; Lamb, C.A.; Feagan, B.G.; Panes, J.; Salas, A.; et al. Etrolizumab as induction therapy for ulcerative colitis: A randomised, controlled, phase 2 trial. Lancet 2014, 384, 309–318. [Google Scholar] [CrossRef] [Green Version]
- A Clinical Trial to Compare Etrolizumab with Placebo and Adalimumab in Patients with Moderate to Severe Ulcerative Colitis Who Have not Received Treatment with Tumour Necrosis Factor Inhibitors (Hibiscus I). Available online: https://forpatients.roche.com/en/trials/autoimmune-disorder/ulcerative-colitis/a-study-comparing-the-efficacy-and-safety-of-etrolizumab-with-ad.html (accessed on 3 January 2023).
- A Clinical Trial to Compare Etrolizumab with Placebo and Adalimumab in Patients with Moderate to Severe Ulcerative Colitis Who Have not Received Treatment with Tumour Necrosis Factor Inhibitors (Hibiscus II). Available online: https://genentech-clinicaltrials.com/en/trials/autoimmune-disorder/ulcerative-colitis/a-study-comparing-the-efficacy-and-safety-of-etrolizumab-with-ad0.html (accessed on 3 January 2023).
- Danese, S.; Colombel, J.F.; Lukas, M.; Gisbert, J.P.; D’Haens, G.; Hayee, B.; Panaccione, R.; Kim, H.S.; Reinisch, W.; Tyrrell, H.; et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): A randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 118–127. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Tyrrell, H.; Hassanali, A.; Lacey, S.; Tole, S.; Tatro, A.R.; Etrolizumab Global Steering Committee. Etrolizumab for the Treatment of Ulcerative Colitis and Crohn’s Disease: An Overview of the Phase 3 Clinical Program. Adv. Ther. 2020, 37, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Hackney, J.A.; Ichikawa, R.; Nguyen, A.; Elstrott, J.; Orozco, L.D.; Sun, K.H.; Modrusan, Z.; Gogineni, A.; Scherl, A.; et al. Dual targeting of lymphocyte homing and retention through α4β7 and αEβ7 inhibition in inflammatory bowel disease. Cell Rep. Med. 2021, 2, 10038. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.B.; Cheng, X.; Mattheakis, L.; Hwang, C.C.; Nawabi, R.; Liu, D.; Gupta, S. Single- and Multiple-Dose Pharmacokinetics and Pharmacodynamics of PN-943, a Gastrointestinal-Restricted Oral Peptide Antagonist of α4β7, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2021, 10, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Mattheakis, L.; Bhandari, A.; Bai, L.; Zemede, G.; Tran, V.; Celino, H.; Frederick, B.; Zhao, L.; Dogra, M.; Lister, H.; et al. P-126 PTG-100, An Oral Peptide Antagonist of Integrin α4β7 that Alters Trafficking of Gut Homing T Cells in Preclinical Animal Models. Inflamm. Bowel Dis. 2016, 22 (Suppl. 1), S48. [Google Scholar] [CrossRef] [Green Version]
- Mattheakis, L.; Fosser, C.; Saralaya, R.; Horsch, K.; Rao, N.; Bai, L.; Zhao, L.; Annamalai, T.; Liu, D. P113 Model based predictions of the PTG-100 pharmacodynamic responses in ulcerative colitis patients. J. Crohns Colitis 2017, 11 (Suppl. 1), S132–S133. [Google Scholar] [CrossRef] [Green Version]
- PN-943 in Adults With Moderate to Severe Active Ulcerative Colitis (UC). Available online: https://clinicaltrials.gov/ct2/show/NCT04504383 (accessed on 13 October 2022).
- Sandborn, W.J.; Lee, S.D.; Tarabar, D.; Louis, E.; Klopocka, M.; Klaus, J.; Reinisch, W.; Hébuterne, X.; Park, D.I.; Schreiber, S.; et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: Report of the OPERA study. Gut 2018, 67, 1824–1835. [Google Scholar] [CrossRef] [Green Version]
- Vermeire, S.; Sandborn, W.J.; Danese, S.; Hébuterne, X.; Salzberg, B.A.; Klopocka, M.; Tarabar, D.; Vanasek, T.; Greguš, M.; Hellstern, P.A.; et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 135–144. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Cyrille, M.; Berner Hansen, M.; Feagan, B.G.; Loftus, E.V.; Vermeire, S.; Cruz, M.L.; Mo, M.; Sullivan, B.A.; Reinisch, W. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J. Crohns Colitis 2017, 11 (Suppl. 1), S22–S23. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Cyrille, M.; Hansen, M.B.; Feagan, B.G.; Loftus, E.; Rogler, G.; Vermeire, S.; Cruz, M.L.; Yang, J.; Boedigheimer, M.J.; et al. Efficacy and Safety of Abrilumab in a Randomized, Placebo-Controlled Trial for Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2019, 156, 946–957.e18. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Kageyama, S.; Andou, A.; Miyazawa, T.; Ejima, C.; Nakayama, A.; Dohi, T.; Eda, H. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J. Crohns Colitis 2013, 7, e533–e542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, N.; Watanabe, M.; Motoya, S.; Tominaga, K.; Matsuoka, K.; Iwakiri, R.; Watanabe, K.; Hibi, T.; AJM300 Study Group. Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 2015, 149, 1775–1783.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takazoe, M.; Watanabe, M.; Kawaguchi, T.; Matsumoto, T.; Oshitani, N.; Hiwatashi, N.; Toshifumi, H. S1066 Oral Alpha-4 Integrin Inhibitor (AJM300) in Patients with Active Crohn’s Disease—A Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology 2009, 5 (Suppl. 1), A-181. [Google Scholar] [CrossRef]
- Fukase, H.; Kajioka, T.; Oikawa, I.; Ikeda, N.; Furuie, H. AJM300, a novel oral antagonist of α4-integrin, sustains an increase in circulating lymphocytes: A randomised controlled trial in healthy male subjects. Br. J. Clin. Pharmacol. 2020, 86, 591–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladrón Abia, P.; Alcalá Vicente, C.; Martínez Delgado, S.; Bastida Paz, G. Fingolimod-induced remission in a patient with ulcerative colitis and multiple sclerosis. Gastroenterol. Hepatol. 2021, 44, 156–157. [Google Scholar] [CrossRef]
- Danese, S.; Furfaro, F.; Vetrano, S. Targeting S1P in Inflammatory Bowel Disease: New Avenues for Modulating Intestinal Leukocyte Migration. J. Crohns Colitis 2018, 12 (Suppl. 2), S678–S686. [Google Scholar] [CrossRef] [Green Version]
- Lassiter, G.; Melancon, C.; Rooney, T.; Murat, A.M.; Kaye, J.S.; Kaye, A.M.; Kaye, R.J.; Cornett, E.M.; Kaye, A.D.; Shah, R.J.; et al. Ozanimod to Treat Relapsing Forms of Multiple Sclerosis: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Neurol. Int. 2020, 12, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohns Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Wolf, D.C.; D’Haens, G.; Vermeire, S.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N. Engl. J. Med. 2016, 374, 1754–1762. [Google Scholar] [CrossRef]
- Dgwshsjigs, S. Ozanimod As Induction Therapy in Moderate-To-Severe Ulcerative Colitis: Results from The Phase 3. Randomized, Double-Blind, Placebo-Controlled True North Study. United Eur. Gastroenterol. J. 2020, 8, 3. [Google Scholar]
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 550–561.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Haens, G.; Danese, S.; Davies, M.; Watanabe, M.; Hibi, T. A phase II, Multicentre, Randomised, Double-Blind, Placebo-controlled Study to Evaluate Safety, Tolerability, and Efficacy of Amiselimod in Patients with Moderate to Severe Active Crohn’s Disease. J. Crohns Colitis 2022, 16, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, K.; Reich, K.; Leonardi, C.L.; Kircik, L.; Chimenti, S.; Langley, R.G.B.; Hu, C.; Stevens, R.M.; Day, R.M.; Gordon, K.B.; et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM) 1). J. Am. Acad. Dermatol. 2015, 73, 37–49. [Google Scholar] [CrossRef]
- Danese, S.; Neurath, M.F.; Kopoń, A.; Zakko, S.F.; Simmons, T.C.; Fogel, R.; Siegel, C.A.; Panaccione, R.; Zhan, X.; Usiskin, K.; et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients With Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2526–2534.e9. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Clotet, A.; Castro-Poceiro, J.; Panés, J. Tofacitinib for the treatment of ulcerative colitis. Expert Rev. Clin. Immunol. 2018, 14, 881–892. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Panés, J.; Vermeire, S.; Lindsay, J.O.; Sands, B.E.; Su, C.; Friedman, G.; Zhang, H.; Yarlas, A.; Bayliss, M.; Maher, S.; et al. Tofacitinib in Patients with Ulcerative Colitis: Health-Related Quality of Life in Phase 3 Randomised Controlled Induction and Maintenance Studies. J. Crohns Colitis 2019, 13, 139–140. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, F.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Tofacitinib in the treatment of ulcerative colitis: Efficacy and safety from clinical trials to real-world experience. Ther. Adv. Gastroenterol. 2019, 12, 48631. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Sharara, A.I.; Su, C.; Modesto, I.; Mundayat, R.; Gunay, L.M.; Salese, L.; Sands, B.E. Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status. Clin. Gastroenterol. Hepatol. 2022, 20, 591–601.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Keam, S.J. Filgotinib in Rheumatoid Arthritis: A Profile of Its Use. Clin. Drug Investig. 2021, 41, 741. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Loftus, E.; Danese, S.; Vermeire, S.; Sandborn, W.J.; Fogel, R.; Nijhawan, S.; Kempinski, R.; Filip, R.; Hospodarskyy, I.; et al. A17 efficacy and safety of filgotinib as maintenance therapy for patients with moderately to severely active ulcerative colitis: Results from the phase 2B/3 selection study. J. Can. Assoc. Gastroenterol. 2021, 4 (Suppl. 1), 21–23. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Upadacitinib for ulcerative colitis. Lancet 2022, 399, 2077–2078. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Loftus, E.V.; van Assche, G.; D’Haens, G.; Schreiber, S.; Colombel, J.F.; Lewis, J.D.; Ghosh, S.; Peyrin-Biroulet, L.; et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology 2020, 158, 2123–2138.e8. [Google Scholar] [CrossRef]
- Parigi, T.L.; Solitano, V.; Peyrin-Biroulet, L.; Danese, S. Do JAK inhibitors have a realistic future in treating Crohn’s disease? Clin. Immunol. 2021, 18, 181–183. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.Y. The Era of Janus Kinase Inhibitors for Inflammatory Bowel Disease Treatment. Int. J. Mol. Sci. 2021, 22, 11322. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Feagan, B.G.; Lichtenstein, G.R.; Zhang, H.; Strauss, R.; Szapary, P.; Johanns, J.; Panes, J.; Vermeire, S.; et al. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-severe Ulcerative Colitis: Results From a Randomised, Phase 2 Study. J. Crohns Colitis 2018, 12, 1158–1169. [Google Scholar] [CrossRef]
- Beattie, D.T.; Pulido-Rios, M.T.; Shen, F.; Ho, M.; Situ, E.; Tsuruda, P.R.; Brassil, P.; Kleinschek, M.; Hegde, S. Intestinally-restricted Janus Kinase inhibition: A potential approach to maximize the therapeutic index in inflammatory bowel disease therapy. J. Inflamm. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Nguyen, D.D.; Beattie, D.T.; Brassil, P.; Krey, W.; Woo, J.; Situ, E.; Sana, R.; Sandvik, E.; Pulido-Rios, M.; et al. Development of Gut-Selective Pan-Janus Kinase Inhibitor TD-1473 for Ulcerative Colitis: A Translational Medicine Programme. J. Crohns Colitis 2020, 14, 1202–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theravance’s Izencitinib Fails in Phase IIb Ulcerative Colitis Trial. Available online: https://www.clinicaltrialsarena.com/news/theravance-izencitinib-ulcerative-colitis/ (accessed on 23 October 2022).
- Muromoto, R.; Oritani, K.; Matsuda, T. Current understanding of the role of tyrosine kinase 2 signaling in immune responses. World J. Biol. Chem. 2022, 13, 1. [Google Scholar] [CrossRef]
- Nogueira, M.; Puig, L.; Torres, T. JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- NCT03395184. Study To Evaluate The Efficacy and Safety of Oral PF-06651600 and PF 06700841 in Subjects with Moderate to Severe Crohn’s Disease. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01559550/full (accessed on 8 January 2023).
- Sandborn, W.; Danese, S.; Leszczyszyn, J.; Romatowski, J.; Altintas, E.; Peeva, E.; Vincent, M.; Reddy, P.; Banfield, C.; Banerjee, A.; et al. OP33 Oral ritlecitinib and brepocitinib in patients with Moderate to Severe Active Ulcerative Colitis: Data from the VIBRATO umbrella study. J. Crohns Colitis 2021, 15 (Suppl. 1), S030–S031. [Google Scholar] [CrossRef]
- Mease, P.J.; Deodhar, A.A.; van der Heijde, D.; Behrens, F.; Kivitz, A.J.; Neal, J.; Kim, J.; Singhal, S.; Nowak, M.; Banerjee, S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022, 81, 815–822. [Google Scholar] [CrossRef] [PubMed]
- EUCTR2019-004878-26-NL. A Study of the Safety, Efficacy, and Biomarker Response of BMS-986165 in Participants with Moderate to Severe Ulcerative Colitis. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02256022/full (accessed on 23 October 2022).
- Bristol Myers Squibb-Bristol Myers Squibb Provides Update on Phase 2 Study of Deucravacitinib in Patients with Moderate to Severe Ulcerative Colitis. Available online: https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Provides-Update-on-Phase-2-Study-of-Deucravacitinib-in-Patients-With-Moderate-to-Severe-Ulcerative-Colitis/default.aspx (accessed on 23 October 2022).
- Atreya, R.; Bloom, S.; Scaldaferri, F.; Gerardi, V.; Admyre, C.; Karlsson, Å.; Knittel, T.; Kowalski, J.; Lukas, M.; Löfberg, R.; et al. Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis. J. Crohns Colitis 2016, 10, 1294. [Google Scholar] [CrossRef] [Green Version]
- Atreya, R.; Peyrin-Biroulet, L.; Klymenko, A.; Augustyn, M.; Bakulin, I.; Slankamenac, D.; Miheller, P.; Gasbarrini, A.; Hébuterne, X.; Arnesson, K.; et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): A phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol. Hepatol. 2020, 5, 1063–1075. [Google Scholar] [CrossRef]
- Study Record|Beta. Available online: https://beta.clinicaltrials.gov/study/NCT04985968 (accessed on 8 January 2023).


| Class | Drug | Target | Route of Administration | Clinical Trial UC CD | |
|---|---|---|---|---|---|
| JAK | Tofacitinib | JAK1/JAK3 | Oral | FDA Approved | Phase IIb |
| Filgotinib | JAK1 | Oral | Phase III | Phase III | |
| Upadacitinib | JAK1 | Oral | FDA Approved | Phase III | |
| Izencitinib | JAK1 | Oral | Phase IIb | ||
| Peficitinib | JAK1 | Oral | Phase IIb | ||
| Ritlecitinib | JAK1 | Oral | Umbrella Study | ||
| JAK/TYK2 | Brepocitinib | TYK2/JAK1 | Oral | Umbrella Study | |
| PDE | Apremilast | PDE4 | Oral | Phase II | |
| Anti-IL-23 | Risankizumab | IL23/p19 subunit | IV or SC | Phase III | Phase III |
| Brazikumab | IL23/p19 subunit | IV or SC | Phase II/OLE | Phase IIb/III | |
| Mirikizumab | IL23/p19 subunit | IV or SC | Phase II | ||
| Guselkumab | IL23/p19 subunit | IV or SC | Phase IIb/III | Phase II/III | |
| Anti-adhesion Molecules | Etrolizumab | α4β7 integrin | SC | Phase I | Phase I |
| PN-943 | α4β7 integrin | Oral | Phase I | ||
| Vedolizumab | α4β7 integrin | IV | Phase IV | Phase IV | |
| AJM300 | α4 integrin | Oral | Phase III | Phase III | |
| PF-00547659 | MAdCAM | SC | Phase II completed | Phase II completed | |
| S1P receptor modulators | Ozanimod | S1P1 and S1P5 receptors | Oral | Phase IV | Phase IV |
| Phase II/III | Phase II/III | ||||
| Etrasimod | S1P1, S1P4, and S1P5 receptors | Oral | Phase II | Phase II/III | |
| Amiselimod | SIP, S1PR1 | Oral | Phase II | Phase II | |
| TLR9 agonist | Cobitolimod | TLR9 | Topical | Phase IIb completed/Phase III under process | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. https://doi.org/10.3390/biomedicines11030747
Zurba Y, Gros B, Shehab M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines. 2023; 11(3):747. https://doi.org/10.3390/biomedicines11030747
Chicago/Turabian StyleZurba, Yasmin, Beatriz Gros, and Mohammad Shehab. 2023. "Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review" Biomedicines 11, no. 3: 747. https://doi.org/10.3390/biomedicines11030747