Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension
Abstract
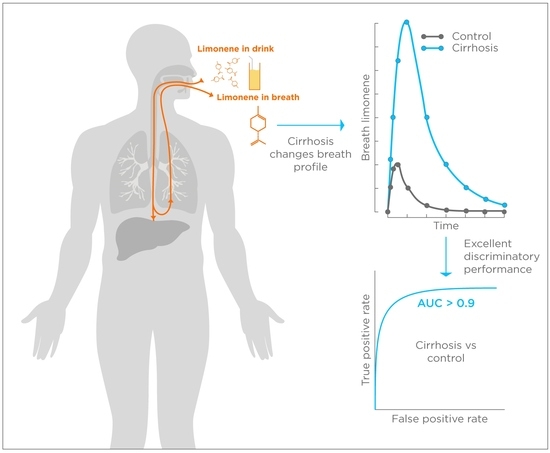
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Breath Biopsy Collection
2.3. Limonene Measurements
2.4. Data Handling and Statistical Analysis
3. Results
3.1. Subjects Characteristics
3.2. Limonene Exhalation Kinetic
3.3. Limonene Association with Signs of Portal Hypertension
3.4. Limonene Classification Performance
3.5. Correlation of Limonene Breath Test with Severity of Cirrhosis and Potential Use as a Prognostic Tool
3.6. Case Reports
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Holzhutter, H.G.; Wuensch, T.; Gajowski, R.; Berndt, N.; Bulik, S.; Meierhofer, D.; Stockmann, M. A novel variant of the (13)C-methacetin liver function breath test that eliminates the confounding effect of individual differences in systemic CO2 kinetics. Arch. Toxicol. 2020, 94, 401–415. [Google Scholar] [CrossRef]
- Labenz, C.; Arslanow, A.; Nguyen-Tat, M.; Nagel, M.; Worns, M.A.; Reichert, M.C.; Heil, F.J.; Mainz, D.; Zimper, G.; Romer, B.; et al. Structured Early detection of Asymptomatic Liver Cirrhosis: Results of the population-based liver screening program SEAL. J. Hepatol. 2022, 77, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagalli, S. Chronic Liver Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gines, P.; Castera, L.; Lammert, F.; Graupera, I.; Serra-Burriel, M.; Allen, A.M.; Wong, V.W.; Hartmann, P.; Thiele, M.; Caballeria, L.; et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology 2022, 75, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gines, P.; Graupera, I.; Lammert, F.; Angeli, P.; Caballeria, L.; Krag, A.; Guha, I.N.; Murad, S.D.; Castera, L. Screening for liver fibrosis in the general population: A call for action. Lancet Gastroenterol. Hepatol. 2016, 1, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Graupera, I.; Lammert, F. Screening is caring: Community-based non-invasive diagnosis and treatment strategies for hepatitis C to reduce liver disease burden. J. Hepatol. 2018, 69, 562–563. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef]
- Macpherson, I.; Abeysekera, K.W.M.; Harris, R.; Mansour, D.; McPherson, S.; Rowe, I.; Rosenberg, W.; Dillon, J.F.; Yeoman, A.; Specialist Interest Group in the Early Detection of Liver Disease. Identification of liver disease: Why and how. Frontline Gastroenterol. 2022, 13, 367–373. [Google Scholar] [CrossRef]
- Kim, B.K.; Tamaki, N.; Imajo, K.; Yoneda, M.; Sutter, N.; Jung, J.; Lin, T.; Tu, X.M.; Bergstrom, J.; Nguyen, K.; et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J. Hepatol. 2022, 77, 1482–1490. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Foucquier, J.; Younossi, Z.M.; Harrison, S.A.; Newsome, P.N.; Chan, W.K.; Yilmaz, Y.; De Ledinghen, V.; Costentin, C.; Zheng, M.H.; et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J. Hepatol. 2023, 78, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Haworth, J.J.; Pitcher, C.K.; Ferrandino, G.; Hobson, A.R.; Pappan, K.L.; Lawson, J.L.D. Breathing new life into clinical testing and diagnostics: Perspectives on volatile biomarkers from breath. Crit. Rev. Clin. Lab. Sci. 2022, 59, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Mayhew, C.A.; Unterkofler, K. Modelling of Breath and Various Blood Volatilomic Profiles-Implications for Breath Volatile Analysis. Molecules 2022, 27, 2381. [Google Scholar] [CrossRef] [PubMed]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; van der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath. Res. 2019, 13, 032001. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shindo, M.; Shimada, T. Metabolism of (+)- and (−)-limonenes to respective carveols and perillyl alcohols by CYP2C9 and CYP2C19 in human liver microsomes. Drug Metab. Dispos. 2002, 30, 602–607. [Google Scholar] [CrossRef]
- Miyazawa, M.; Marumoto, S.; Takahashi, T.; Nakahashi, H.; Haigou, R.; Nakanishi, K. Metabolism of (+)- and (−)-menthols by CYP2A6 in human liver microsomes. J. Oleo Sci. 2011, 60, 127–132. [Google Scholar] [CrossRef]
- Murgia, A.; Ahmed, Y.; Sweeney, K.; Nicholson-Scott, L.; Arthur, K.; Allsworth, M.; Boyle, B.; Gandelman, O.; Smolinska, A.; Ferrandino, G. Breath-Taking Perspectives and Preliminary Data toward Early Detection of Chronic Liver Diseases. Biomedicines 2021, 9, 1563. [Google Scholar] [CrossRef]
- Hardwick, R.N.; Fisher, C.D.; Street, S.M.; Canet, M.J.; Cherrington, N.J. Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab. Dispos. 2012, 40, 450–460. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Gotze, O.; Geier, A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J. Gastroenterol. 2016, 22, 72–88. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Reichen, J. Pharmacokinetics and Pharmacodynamics in Cirrhosis. Medicine 2002, 30, 24–27. [Google Scholar] [CrossRef]
- Duthaler, U.; Bachmann, F.; Suenderhauf, C.; Grandinetti, T.; Pfefferkorn, F.; Haschke, M.; Hruz, P.; Bouitbir, J.; Krahenbuhl, S. Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel Phenotyping Cocktail Differently. Clin. Pharmacokinet. 2022, 61, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; de Saedeleer, A.; Kibble, G.; et al. Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound-Toward Improved Detection of Liver Impairment. Clin. Transl. Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, K.K.; Budd, T. Pharmacokinetics of d-limonene in the rat by GC-MS assay. J. Pharm. Biomed. Anal. 1998, 17, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sheng, Y.; Hu, Y.; Sun, J.; Li, W.; Feng, H.; Tang, L. Determination of d-limonene in mice plasma and tissues by a new GC-MS/MS method: Comparison of the pharmacokinetics and tissue distribution by oral and inhalation administration in mice. Biomed. Chromatogr. 2019, 33, e4530. [Google Scholar] [CrossRef]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef]
- Fernandez Del Rio, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile Biomarkers in Breath Associated With Liver Cirrhosis—Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef]
- Friedman, M.I.; Preti, G.; Deems, R.O.; Friedman, L.S.; Munoz, S.J.; Maddrey, W.C. Limonene in expired lung air of patients with liver disease. Dig. Dis. Sci. 1994, 39, 1672–1676. [Google Scholar] [CrossRef]
- Pijls, K.E.; Smolinska, A.; Jonkers, D.M.; Dallinga, J.W.; Masclee, A.A.; Koek, G.H.; van Schooten, F.J. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci. Rep. 2016, 6, 19903. [Google Scholar] [CrossRef]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.M.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef]
- The World Medical Association (WMA). World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- European Association for the Study of the Liver; European Organisation for Research; Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Razlan, H.; Marzuki, N.M.; Tai, M.L.; Shamsul, A.S.; Ong, T.Z.; Mahadeva, S. Diagnostic value of the C methacetin breath test in various stages of chronic liver disease. Gastroenterol. Res. Pract. 2011, 2011, 235796. [Google Scholar] [CrossRef]
- Ferrandino, G.; De Palo, G.; Murgia, A.; Birch, O.; Tawfike, A.; Smith, R.; Debiram-Beecham, I.; Gandelman, O.; Kibble, G.; Lydon, A.M.; et al. Breath Biopsy((R)) to Identify Exhaled Volatile Organic Compounds Biomarkers for Liver Cirrhosis Detection. J. Clin. Transl. Hepatol. 2023, 11, 638–648. [Google Scholar] [CrossRef]
- Python Software Foundation. Python Language Reference, Version 2.7. Available online: http://www.python.org (accessed on 15 March 2023).
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 15 March 2023).
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 1–4. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix. Available online: https://github.com/taiyun/corrplo (accessed on 15 March 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer, Ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Denney, W.; Duvvuri, S.; Buckeridge, C. Simple, Automatic Noncompartmental Analysis: The PKNCA R Package. J. Pharmacokinet. Pharmacodyn. 2015, 41, 11–107. [Google Scholar] [CrossRef]
- Smolinska, A.; Tedjo, D.I.; Blanchet, L.; Bodelier, A.; Pierik, M.J.; Masclee, A.A.M.; Dallinga, J.; Savelkoul, P.H.M.; Jonkers, D.; Penders, J.; et al. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal. Chim. Acta 2018, 1025, 1–11. [Google Scholar] [CrossRef]
- Iwakiri, Y. Pathophysiology of portal hypertension. Clin. Liver Dis. 2014, 18, 281–291. [Google Scholar] [CrossRef]
- Maruyama, H.; Yokosuka, O. Ultrasonography for Noninvasive Assessment of Portal Hypertension. Gut Liver 2017, 11, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; et al. Rapid “breath-print” of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS ONE 2013, 8, e59658. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.E.; Fernandez Del Rio, R.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Limonene in exhaled breath is elevated in hepatic encephalopathy. J. Breath. Res. 2016, 10, 046010. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, G.; van Munster, K.; Ferrandino, G.; Sauca, M.; Ponsioen, C.; van Schooten, F.J.; Smolinska, A. Liver Impairment-The Potential Application of Volatile Organic Compounds in Hepatology. Metabolites 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Har-Shai, L.; Jeries, R.; Cancilla, J.C.; Glass-Marmor, L.; Lejbkowicz, I.; Torrecilla, J.S.; Yao, X.; Feng, X.; Narita, A.; et al. Exhaled Breath Markers for Nonimaging and Noninvasive Measures for Detection of Multiple Sclerosis. ACS Chem. Neurosci. 2017, 8, 2402–2413. [Google Scholar] [CrossRef]
- Saltzman, A.; Caraway, W.T. Cinnamic acid as a test substance in the evaluation of liver function. J. Clin. Investig. 1953, 32, 711–719. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Miller, J.A.; Hakim, I.A.; Chew, W.; Thompson, P.; Thomson, C.A.; Chow, H.H. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutr. Cancer 2010, 62, 783–788. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Yan, J.; Kang, Y.; Xu, S.; Ong, L.L.; Zhuo, S.; Bunte, R.M.; Chen, N.; Asada, H.H.; So, P.T.; Wanless, I.R.; et al. In vivo label-free quantification of liver microcirculation using dual-modality microscopy. J. Biomed. Opt. 2014, 19, 116006. [Google Scholar] [CrossRef]
- Nazir, N.U.; Abbas, S.R.; Nasir, H.; Hussain, I. Electrochemical sensing of limonene using thiol capped gold nanoparticles and its detection in the real breath sample of a cirrhotic patient. J. Electroanal. Chem. 2022, 905, 115977. [Google Scholar] [CrossRef]
- Weber, I.C.; Oosthuizen, D.N.; Mohammad, R.W.; Mayhew, C.A.; Pratsinis, S.E.; Guntner, A.T. Dynamic Breath Limonene Sensing at High Selectivity. ACS Sens. 2023, 8, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Cowan, J.M. Reflections on variability in the blood-breath ratio of ethanol and its importance when evidential breath-alcohol instruments are used in law enforcement. Forensic Sci. Res. 2020, 5, 300–308. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br. J. Surg. 2018, 105, 1493–1500. [Google Scholar] [CrossRef] [PubMed]





| Control | Cirrhosis | p-Values | |
|---|---|---|---|
| Number of patients | 29 | 29 | |
| Age median [IQR] Years | 43 [38–57] | 59 [54–67] | <0.001 |
| Sex, n (%) | |||
| Male | 11 (38%) | 9 (31%) | |
| Female | 18 (62%) | 20 (69%) | |
| Height median [IQR] cm | 163 [158–170] | 160 [156–170] | 0.32 |
| Weight median [IQR] kg | 75 [64–84] | 78 [68–85] | 0.50 |
| BMI median [IQR] | 26.8 [23.9–31.2] | 27.7 [26.0–32.8] | 0.23 |
| Waist circumference median [IQR] (cm) | 90 [81.5–104] | 106 [97–113] | 0.036 |
| Child–Pugh class | - | ||
| A | 23 (78%) | ||
| B | 5 (16%) | ||
| N/A | 2 (6%) | ||
| MELD median [IQR] | - | 10 [7.2–12.8] | |
| FIB4 median [IQR] | 1.4 [0.8–3.1] | 2.3 [1.9–4] | p < 0.001 |
| APRI median [IQR] | 0.2 [0.2–0.3] | 0.6 [0.4–0.9] | p < 0.001 |
| Platelets median [IQR] ×109/L | 240 [216–294] | 147 [107–209] | p < 0.001 |
| Total bilirubin median [IQR] (µmol/L) | 8.2 [6.5–12.9] | 14.3 [8.2–18.4] | p < 0.001 |
| Serum albumin median [IQR] (g/L) | 45 [44–45] | 40 [37–44.5] | p < 0.001 |
| INR median [IQR] | 1 [1–1.06] | 1.2 [1.0–1.4] | p < 0.001 |
| ALT median [IQR] (IU/L) | 18 [15–26] | 28.5 [18.7–38] | p < 0.001 |
| AST median [IQR] (IU/L) | 21 [18.5–24] | 35 [27.2–45.7] | p < 0.001 |
| GGT median [IQR] (IU/L) | 24 [16–32] | 76 [59.2–108.7] | p < 0.001 |
| ALP median [IQR] (IU/L) | 85 [71.5–101.5] | 117 [94.5–153] | p < 0.001 |
| Creatinine median [IQR] (mg/dL) | 0.78 [0.68–1.93] | 0.74 [0.64–0.87] | 0.31 |
| Sodium median [IQR] (mM) | 141 [138.7–142.5] | 142 [139.5–142.5] | 0.65 |
| Parameter | Control | Cirrhosis | p-Value |
|---|---|---|---|
| Cmax (ng) median [IQR] | 595 [361–903] | 2077 [1051–4260] | <0.001 |
| Log10 C0 (ng) median [IQR] | 6.9 [6.69–7.29] | 8.4 [7.9–8.9] | <0.001 |
| Tmax, n (%) | |||
| 20 min | 18 (62.1%) | 13 (44.8%) | |
| 40 min | 11 (37.9%) | 12 (41.4%) | |
| 60 min | 0 | 2 (6.9%) | |
| 90 min | 0 | 1 (3.4%) | |
| 120 min | 0 | 1 (3.4%) | |
| AUC (0–90 min) ng × min/400 mL median [IQR] | 27,107 [17,605–34,946] | 121,437 [57,921–202,733] | <0.001 |
| Slope | −0.027 [−0.031–−0.023] | −0.025 [−0.027–0.019] | 0.072 |
| Timepoint (min) | AUROC | Sensitivity/Specificity | +/− Predictive Values (%) | +/− Likelihood Ratios |
|---|---|---|---|---|
| 0 | 0.83 ± 0.12 | 0.66 ± 0.09/ 0.83 ± 0.07 | 79.17/70.59 | 3.8/0.42 |
| 20 | 0.92 ± 0.07 | 0.82 ± 0.095/ 0.79 ± 0.08 | 79.88/81.62 | 3.97/0.23 |
| 40 | 0.94 ± 0.06 | 0.79 ± 0.08/ 0.9 ± 0.06 | 88.37/80.71 | 7.6/0.24 |
| 60 | 0.91 ± 0.07 | 0.83 ± 0.07/ 0.9 ± 0.06 | 88.89/83.87 | 8.0/0.19 |
| 90 | 0.91 ± 0.07 | 0.76 ± 0.08/ 0.86 ± 0.06 | 84.62/78.12 | 5.5/0.28 |
| 120 | 0.93 ± 0.06 | 0.83 ± 0.07/ 0.86 ± 0.06 | 85.71/83.33 | 6.0/0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrandino, G.; Ricciardi, F.; Murgia, A.; Banda, I.; Manhota, M.; Ahmed, Y.; Sweeney, K.; Nicholson-Scott, L.; McConville, L.; Gandelman, O.; et al. Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines 2023, 11, 2957. https://doi.org/10.3390/biomedicines11112957
Ferrandino G, Ricciardi F, Murgia A, Banda I, Manhota M, Ahmed Y, Sweeney K, Nicholson-Scott L, McConville L, Gandelman O, et al. Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines. 2023; 11(11):2957. https://doi.org/10.3390/biomedicines11112957
Chicago/Turabian StyleFerrandino, Giuseppe, Federico Ricciardi, Antonio Murgia, Iris Banda, Menisha Manhota, Yusuf Ahmed, Kelly Sweeney, Louise Nicholson-Scott, Lucinda McConville, Olga Gandelman, and et al. 2023. "Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension" Biomedicines 11, no. 11: 2957. https://doi.org/10.3390/biomedicines11112957