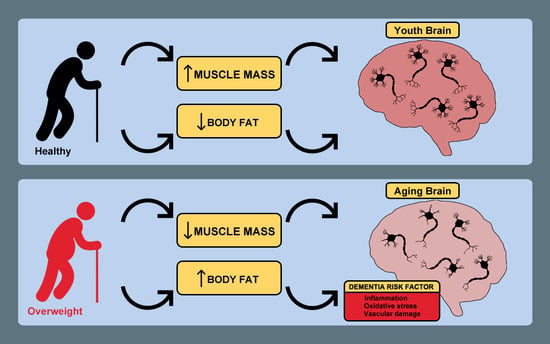
Poor Cognitive Agility Conservation in Obese Aging People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Groups
2.3. Anthropometric Measurements and Bioelectrical Impedance Analysis
2.4. Statistical Analysis and Correlation Studies
2.5. Ethical Aspects of Research
3. Results
3.1. Body Height, Body Weight and Basal Metabolic Rate
3.2. Body Composition
3.3. Morphological Composition and with Mental Agility Conservation Correlation
4. Discussion
Study Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyul-Toth, A.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A.; et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metab. Clin. Exp. 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Fernandez-Molina, M.; Singh, G.; Correa, R. Obesity and its cardiovascular effects. Diabetes/Metab. Res. Rev. 2019, 35, e3135. [Google Scholar] [CrossRef]
- Gustafson, D.; Rothenberg, E.; Blennow, K.; Steen, B.; Skoog, I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003, 163, 1524–1528. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhao, W.; Lu, M.; Zhang, X.; Zhang, P.; Xin, Z.; Sun, R.; Tian, W.; Cardoso, M.A.; Yang, J.; et al. Relationship between Central Obesity and the incidence of Cognitive Impairment and Dementia from Cohort Studies Involving 5,060,687 Participants. Neurosci. Biobehav. Rev. 2021, 130, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Sala, L.; Pontiroli, A.E. Role of obesity and hypertension in the incidence of atrial fibrillation, ischaemic heart disease and heart failure in patients with diabetes. Cardiovasc. Diabetol. 2021, 20, 162. [Google Scholar] [CrossRef]
- Bilotta, F.; Lauretta, M.P.; Tewari, A.; Haque, M.; Hara, N.; Uchino, H.; Rosa, G. Insulin and the Brain: A Sweet Relationship With Intensive Care. J. Intensive Care Med. 2017, 32, 48–58. [Google Scholar] [CrossRef]
- Chen, W.; Cai, W.; Hoover, B.; Kahn, C.R. Insulin action in the brain: Cell types, circuits, and diseases. Trends Neurosci. 2022, 45, 384–400. [Google Scholar] [CrossRef]
- Jha, N.K.; Jha, S.K.; Kumar, D.; Kejriwal, N.; Sharma, R.; Ambasta, R.K.; Kumar, P. Impact of Insulin Degrading Enzyme and Neprilysin in Alzheimer’s Disease Biology: Characterization of Putative Cognates for Therapeutic Applications. J. Alzheimer’s Dis. JAD 2015, 48, 891–917. [Google Scholar] [CrossRef] [PubMed]
- Bolzenius, J.D.; Laidlaw, D.H.; Cabeen, R.P.; Conturo, T.E.; McMichael, A.R.; Lane, E.M.; Heaps, J.M.; Salminen, L.E.; Baker, L.M.; Gunstad, J.; et al. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging Behav. 2013, 7, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Cheke, L.G.; Simons, J.S.; Clayton, N.S. Higher body mass index is associated with episodic memory deficits in young adults. Q. J. Exp. Psychol. 2016, 69, 2305–2316. [Google Scholar] [CrossRef] [Green Version]
- Cournot, M.; Marquie, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; Ferrieres, J.; Ruidavets, J.B. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006, 67, 1208–1214. [Google Scholar] [CrossRef]
- Coppin, G.; Nolan-Poupart, S.; Jones-Gotman, M.; Small, D.M. Working memory and reward association learning impairments in obesity. Neuropsychologia 2014, 65, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Conforto, R.M.; Gershman, L. Cognitive processing differences between obese and nonobese subjects. Addict. Behav. 1985, 10, 83–85. [Google Scholar] [CrossRef]
- Hughes, T.F.; Borenstein, A.R.; Schofield, E.; Wu, Y.; Larson, E.B. Association between late-life body mass index and dementia: The Kame Project. Neurology 2009, 72, 1741–1746. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, Y.; Park, S.M. Body Mass Index and Decline of Cognitive Function. PLoS ONE 2016, 11, e0148908. [Google Scholar] [CrossRef]
- Espeland, M.A.; Yassine, H.; Hayden, K.D.; Hugenschmidt, C.; Bennett, W.L.; Chao, A.; Neiberg, R.; Kahn, S.E.; Luchsinger, J.A.; Action for Health in Diabetes Research, G. Sex-related differences in cognitive trajectories in older individuals with type 2 diabetes and overweight or obesity. Alzheimer’s Dement. 2021, 7, e12160. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.N.; Park, D.C. Obesity and Aging: Consequences for Cognition, Brain Structure, and Brain Function. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Solomon, A.; Ahtiluoto, S.; Ngandu, T.; Lehtisalo, J.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.; Ngandu, T.; Liu, Y.; Peltonen, M.; Antikainen, R.; Kemppainen, N.; Laatikainen, T.; Lotjonen, J.; Rinne, J.; Strandberg, T.; et al. Change in CAIDE Dementia Risk Score and Neuroimaging Biomarkers During a 2-Year Multidomain Lifestyle Randomized Controlled Trial: Results of a Post-Hoc Subgroup Analysis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Korkki, S.; Solomon, A.; Coley, N.; Antikainen, R.; Backman, L.; Hanninen, T.; Lindstrom, J.; Laatikainen, T.; et al. The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 1325–1334. [Google Scholar] [CrossRef]
- Peña-Casanova, J.; Aguilar, M.; Bertran-Serra, I.; Santacruz, P.; Hernández, G.; Insa, R.; Pujol, A.; Sol, J.M.; Blesa, R. [Normalization of cognitive and functional assessment instruments for dementia (NORMACODEM) (I): Objectives, content and population]. Neurol. (Barc. Spain) 1997, 12, 61–68. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Brtková, M.; Bakalár, P.; Matúš, I.; Hančová, M.; Rimárová, K. Body composition of undergraduates—Comparison of four different measurement methods. Phys. Act. Rev. 2014, 2, 38–44. [Google Scholar]
- Madden, K.M.; Feldman, B.; Arishenkoff, S.; Meneilly, G.S. A rapid point-of-care ultrasound marker for muscle mass and muscle strength in older adults. Age Ageing 2021, 50, 505–510. [Google Scholar] [CrossRef]
- Garcia-Morales, V.; Gonzalez-Acedo, A.; Melguizo-Rodriguez, L.; Pardo-Moreno, T.; Costela-Ruiz, V.J.; Montiel-Troya, M.; Ramos-Rodriguez, J.J. Current Understanding of the Physiopathology, Diagnosis and Therapeutic Approach to Alzheimer’s Disease. Biomedicines 2021, 9, 1910. [Google Scholar] [CrossRef]
- Hyun, J.; Hall, C.B.; Katz, M.J.; Derby, C.A.; Lipnicki, D.M.; Crawford, J.D.; Guaita, A.; Vaccaro, R.; Davin, A.; Kim, K.W.; et al. Education, Occupational Complexity, and Incident Dementia: A COSMIC Collaborative Cohort Study. J. Alzheimer’s Dis. JAD 2022, 85, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Jimenez-Palomares, M.; Murillo-Carretero, M.I.; Infante-Garcia, C.; Berrocoso, E.; Hernandez-Pacho, F.; Lechuga-Sancho, A.M.; Cozar-Castellano, I.; Garcia-Alloza, M. Central vascular disease and exacerbated pathology in a mixed model of type 2 diabetes and Alzheimer’s disease. Psychoneuroendocrinology 2015, 62, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Ortiz, O.; Jimenez-Palomares, M.; Kay, K.R.; Berrocoso, E.; Murillo-Carretero, M.I.; Perdomo, G.; Spires-Jones, T.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 2013, 38, 2462–2475. [Google Scholar] [CrossRef]
- Zhu, J.; Ge, F.; Zeng, Y.; Qu, Y.; Chen, W.; Yang, H.; Yang, L.; Fang, F.; Song, H. Physical and Mental Activity, Disease Susceptibility, and Risk of Dementia: A Prospective Cohort Study Based on UK Biobank. Neurology 2022, 99, e799–e813. [Google Scholar] [CrossRef] [PubMed]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Doherty, M.G.; Cairns, K.; O’Neill, V.; Lamrock, F.; Jorgensen, T.; Brenner, H.; Schottker, B.; Wilsgaard, T.; Siganos, G.; Kuulasmaa, K.; et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: Results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur. J. Epidemiol. 2016, 31, 455–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, P.K.; Esko, T.; Mattsson, H.; Eklund, N.; Gandin, I.; Nutile, T.; Jackson, A.U.; Schurmann, C.; Smith, A.V.; Zhang, W.; et al. Directional dominance on stature and cognition in diverse human populations. Nature 2015, 523, 459–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, V.H.; Costa, P.S.; Santos, N.C.; Cunha, P.G.; Correia-Neves, M.; Palha, J.A.; Sousa, N. Adult Body Height Is a Good Predictor of Different Dimensions of Cognitive Function in Aged Individuals: A Cross-Sectional Study. Front. Aging Neurosci. 2016, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, N.C.; Costa, P.S.; Cunha, P.; Portugal-Nunes, C.; Amorim, L.; Cotter, J.; Cerqueira, J.J.; Palha, J.A.; Sousa, N. Clinical, physical and lifestyle variables and relationship with cognition and mood in aging: A cross-sectional analysis of distinct educational groups. Front. Aging Neurosci. 2014, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, T.S.H.; Okholm, G.T.; Christensen, K.; Sørensen, T.I.; Osler, M. Body height in young adult men and risk of dementia later in adult life. eLife 2020, 9, e51168. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.K.; Zhan, Y.; Gatz, M.; Reynolds, C.A.; Dahl Aslan, A.K. Change in cognition and body mass index in relation to preclinical dementia. Alzheimer’s Dement. 2021, 7, e12176. [Google Scholar] [CrossRef]
- Kocelak, P.; Mossakowska, M.; Puzianowska-Kuznicka, M.; Sworczak, K.; Wyszomirski, A.; Handzlik, G.; Stefanski, A.; Zdrojewski, T.; Chudek, J. Prevalence and risk factors of untreated thyroid dysfunctions in the older Caucasian adults: Results of PolSenior 2 survey. PloS ONE 2022, 17, e0272045. [Google Scholar] [CrossRef] [PubMed]
- Risal, P.; Adhikari, B.; Shrestha, R.; Manandhar, S.; Bhatt, R.D.; Hada, M. Analysis of Factors Associated with Thyroid Dysfunction: A Hospital Based Study. Kathmandu Univ. Med. J. 2019, 17, 88–92. [Google Scholar]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef]
- Elsworthy, R.J.; Aldred, S. The effect of age and obesity on platelet amyloid precursor protein processing and plasma markers of oxidative stress and inflammation. Exp. Gerontol. 2020, 132, 110838. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Bisht, K.; Sharma, K.; Tremblay, M.E. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spauwen, P.J.; Murphy, R.A.; Jónsson, P.V.; Sigurdsson, S.; Garcia, M.E.; Eiriksdottir, G.; van Boxtel, M.P.; Lopez, O.L.; Gudnason, V.; Harris, T.B.; et al. Associations of fat and muscle tissue with cognitive status in older adults: The AGES-Reykjavik Study. Age Ageing 2017, 46, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Seo, S.; Kim, W.R.; Kim, C.; Noh, Y. Association Between Visceral Fat and Brain Cortical Thickness in the Elderly: A Neuroimaging Study. Front. Aging Neurosci. 2021, 13, 694629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Sun, Y.; Ma, S.; Zhang, K.; Tang, X.; Chen, A. Differences in energy metabolism and mitochondrial redox status account for the differences in propensity for developing obesity in rats fed on high-fat diet. Food Sci. Nutr. 2021, 9, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Fukuhara, A.; Hashimoto, E.; Kobayashi, H.; Kobayashi, S.; Otsuki, M.; Shimomura, I. Oxidative Stress Inhibits Healthy Adipose Expansion Through Suppression of SREBF1-Mediated Lipogenic Pathway. Diabetes 2018, 67, 1113–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Pozo, A.; Growdon, J.H. Is Alzheimer’s Disease Risk Modifiable? J. Alzheimer’s Dis. JAD 2019, 67, 795–819. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Hepple, R.T. Impact of aging on mitochondrial function in cardiac and skeletal muscle. Free Radic. Biol. Med. 2016, 98, 177–186. [Google Scholar] [CrossRef]
- Hepple, R.T. Sarcopenia—A critical perspective. Sci. Aging Knowl. Environ. SAGE KE 2003, 2003, pe31. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.L.; Krause, D.J.; Baker, D.J.; Fu, M.H.; Tarnopolsky, M.A.; Hepple, R.T. Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 1099–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekkers, I.A.; Jansen, P.R.; Lamb, H.J. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology 2019, 292, 270. [Google Scholar] [CrossRef] [PubMed]
- Rajan, L.; McKay, C.C.; Santos Malave, G.; Pearce, A.L.; Cherry, J.B.C.; Mackey, E.; Nadler, E.P.; Vaidya, C.J. Effects of severe obesity and sleeve gastrectomy on cortical thickness in adolescents. Obesity 2021, 29, 1516–1525. [Google Scholar] [CrossRef]
- Li, L.; Zang, L.; Zhang, F.; Chen, J.; Shen, H.; Shu, L.; Liang, F.; Feng, C.; Chen, D.; Tao, H.; et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum. Mol. Genet. 2017, 26, 2398–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Rodriguez, J.J.; Molina-Gil, S.; Ortiz-Barajas, O.; Jimenez-Palomares, M.; Perdomo, G.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PloS ONE 2014, 9, e89229. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Bujalance, C.; Del Marco, A.; Jose Ramos-Rodriguez, J.; Infante-Garcia, C.; Bella Gomez-Santos, S.; Herrera, M.; Garcia-Alloza, M. Cell proliferation and neurogenesis alterations in Alzheimer’s disease and diabetes mellitus mixed murine models. J. Neurochem. 2020, 154, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]



| Groups | Ages | Female (n) | Male (n) | Total (n) |
|---|---|---|---|---|
| G+95% | 73.87 ± 9.249 | 10 | 10 | 19 |
| G95 − 90% | 73.58 ± 9.097 | 12 | 14 | 26 |
| G − 90% | 82.2 ± 2.38 | 8 | 6 | 15 |
| Groups | Body Height | Body Weight | BMI | Basal Metabolic Rate |
|---|---|---|---|---|
| G + 95% | 165.69 ± 9.36 cm | 69.49 ± 15.39 Kg | 25.08 ± 0.98 | 1454.38 ± 253.22 Kcal/day |
| G95 − 90% | 166.53 ± 8.21 cm | 84.35 ** ± 12.56 Kg | 30.61 ** ± 0.72 | 1570.53 ± 223.23 Kcal/day |
| G − 90% | 155.70 ** ± 7.78 cm | 71.98 ± 8.83 Kg | 29.64 ** ± 0.93 | 1389.6 * ± 164.99 Kcal/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Moreno, T.; Mohamed-Mohamed, H.; Rivas-Dominguez, A.; Garcia-Morales, V.; Garcia-Lara, R.A.; Suleiman-Martos, S.; Bermudez-Pulgarin, B.; Ramos-Rodriguez, J.J. Poor Cognitive Agility Conservation in Obese Aging People. Biomedicines 2023, 11, 138. https://doi.org/10.3390/biomedicines11010138
Pardo-Moreno T, Mohamed-Mohamed H, Rivas-Dominguez A, Garcia-Morales V, Garcia-Lara RA, Suleiman-Martos S, Bermudez-Pulgarin B, Ramos-Rodriguez JJ. Poor Cognitive Agility Conservation in Obese Aging People. Biomedicines. 2023; 11(1):138. https://doi.org/10.3390/biomedicines11010138
Chicago/Turabian StylePardo-Moreno, Teresa, Himan Mohamed-Mohamed, Antonio Rivas-Dominguez, Victoria Garcia-Morales, Ruben A. Garcia-Lara, Sami Suleiman-Martos, Beatriz Bermudez-Pulgarin, and Juan Jose Ramos-Rodriguez. 2023. "Poor Cognitive Agility Conservation in Obese Aging People" Biomedicines 11, no. 1: 138. https://doi.org/10.3390/biomedicines11010138