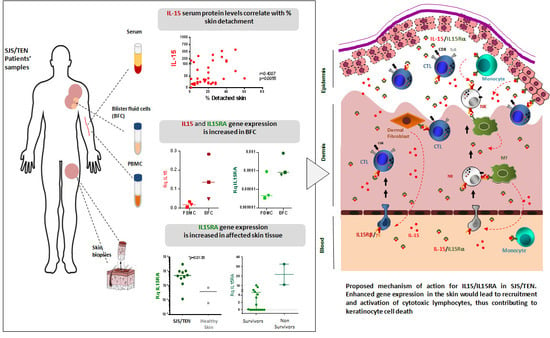
IL-15/IL-15Rα in SJS/TEN: Relevant Expression of IL15 and IL15RA in Affected Skin
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients and Patients’ Samples
2.2. IL-15 Protein Quantification by ELISA
2.3. Real-Time Quantitative RT-PCR
2.4. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. IL-15 Serum Levels in SJS/TEN
3.3. IL-15 Protein Levels in Blister Fluid from SJS/TEN Patients
3.4. Gene Expression Levels of IL15 and IL15RA in PBMCs and BFCs from Patients with SJS/TEN
3.5. Gene Expression Levels of IL15 and IL15RA in CD14+ Monocytes Isolated from PBMCs and BFCs from Patients with SJS/TEN
3.6. Gene Expression Levels of IL15 and IL15RA in Skin Biopsies from Patients with SJS/TEN and Healthy Donors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastuji-Garin, S.; Rzany, B.; Stern, R.S.; Shear, N.H.; Naldi, L.; Roujeau, J.C. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch. Dermatol. 1993, 129, 92–96. [Google Scholar] [CrossRef]
- Paulmann, M.; Mockenhaupt, M. Fever in Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis in Pediatric Cases. Pediatr. Infect. Dis. J. 2017, 36, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Mockenhaupt, M. Epidemiology of cutaneous adverse drug reactions. In Adverse Cutaneous Drug Eruptions; French, L.E., Ed.; Karger: Basel, Switzerland, 2012; Volume 97, pp. 1–17. [Google Scholar] [CrossRef]
- Sekula, P.; Dunant, A.; Mockenhaupt, M.; Naldi, L.; Bavinck, J.N.B.; Halevy, S.; Kardaun, S.; Sidoroff, A.; Liss, Y.; Schumacher, M.; et al. Comprehensive Survival Analysis of a Cohort of Patients with Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2013, 133, 1197–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoetzenecker, W.; Nägeli, M.; Mehra, E.T.; Jensen, A.N.; Saulite, I.; Schmid-Grendelmeier, P.; Guenova, E.; Cozzio, A.; French, L.E. Adverse cutaneous drug eruptions: Current understanding. Semin. Immunopathol. 2016, 38, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dodiuk-Gad, R.P.; Hung, S.-I.; Valeyrie-Allanore, L.; Shear, N.H. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am. J. Clin. Dermatol. 2015, 16, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bavinck, J.N.B.; Sidoroff, A.; Schneck, J.; Roujeau, J.-C.; Flahault, A.; et al. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study. J. Investig. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Le Louet, H. ALDEN, an Algorithm for Assessment of Drug Causality in Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Comparison with Case–Control Analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T. Mechanisms of Severe Cutaneous Adverse Reactions: Recent Advances. Drug Saf. 2019, 42, 973–992. [Google Scholar] [CrossRef]
- Le Cleach, L.; Delaire, S.; Boumsell, L.; Bagot, M.; Bourgault-Villada, I.; Bensussan, A.; Roujeau, J.C. Blister fluid T lymphocytes during toxic epidermal necrolysis are functional cytotoxic cells which express human natural killer (NK) inhibitory receptors. Clin. Exp. Immunol. 2000, 119, 225–230. [Google Scholar] [CrossRef]
- Phillips, E.J. New strategies to predict and prevent serious immunologically mediated adverse drug reactions. Trans. Am. Clin. Clim. Assoc. 2018, 129, 74–87. [Google Scholar]
- Yang, Y.; Li, F.; Du, J.; Shen, Y.; Lin, J.; Zhu, X.; Luo, X.; Liang, J.; Xu, J. Variable levels of apoptotic signal-associated cytokines in the disease course of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Australas. J. Dermatol. 2017, 58, e61–e67. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.P.; Rozieres, A.; Bensaid, B.; Eriksson, K.K.; Mosnier, A.; Albert, F.; Mutez, V.; Brassard, O.; Baysal, T.; Tardieu, M.; et al. Massive clonal expansion of polycytotoxic skin and blood CD8 + T cells in patients with toxic epidermal necrolysis. Sci. Adv. 2021, 7, eabe0013. [Google Scholar] [CrossRef] [PubMed]
- Nassif, A.; Bensussan, A.; Bachot, N.; Bagot, M.; Boumsell, L.; Roujeau, J.-C.; Dorothée, G.; Mami-Chouaib, F. Drug Specific Cytotoxic T-Cells in the Skin Lesions of a Patient with Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2002, 118, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.-H.; Hung, S.-I.; Yang, J.-Y.; Su, S.-C.; Huang, S.-P.; Wei, C.-Y.; Chin, S.-W.; Chiou, C.-C.; Chu, S.-C.; Ho, H.-C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Escamochero, S.; Cabañas, R.; Díaz, R.; Fiandor, A.; Bellón, T. CD94/NKG2C is a killer effector molecule in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J. Allergy Clin. Immunol. 2010, 125, 703–710.e8. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef]
- Clayberger, C.; Krensky, A.M. Granulysin. Curr. Opin. Immunol. 2003, 15, 560–565. [Google Scholar] [CrossRef]
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes Induce Migration of Resting Memory CD8 T Cells into Mucosal Tissues. J. Immunol. 2017, 199, 2536–2546. [Google Scholar] [CrossRef] [Green Version]
- Castillo, E.F.; Schluns, K.S. Regulating the immune system via IL-15 transpresentation. Cytokine 2012, 59, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Luan, L.; Patil, N.K.; Sherwood, E.R. Immunobiology of the IL-15/IL-15Rα complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev. 2017, 38, 10–21. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Miljkovic, M.D.; Conlon, K.C. Interleukin-15 (dys)regulation of lymphoid homeostasis: Implications for therapy of autoimmunity and cancer. J. Exp. Med. 2020, 217, e20191062. [Google Scholar] [CrossRef]
- Su, S.-C.; Mockenhaupt, M.; Wolkenstein, P.; Dunant, A.; Le Gouvello, S.; Chen, C.-B.; Chosidow, O.; Valeyrie-Allanore, L.; Bellon, T.; Sekula, P.; et al. Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2017, 137, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Stern, R.S.; Divito, S.J. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Associations, Outcomes, and Pathobiology—Thirty Years of Progress but Still Much to Be Done. J. Investig. Dermatol. 2017, 137, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Sekula, P.; Liss, Y.; Davidovici, B.; Dunant, A.; Roujeau, J.-C.; Kardaun, S.; Naldi, L.; Schumacher, M.; Mockenhaupt, M. Evaluation of SCORTEN on a Cohort of Patients with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Included in the RegiSCAR Study. J. Burn Care Res. 2011, 32, 237–245. [Google Scholar] [CrossRef]
- Mortier, E.; Woo, T.; Advincula, R.; Gozalo, S.; Ma, A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 2008, 205, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-S.; Rha, M.-S.; Shin, E.-C. IFN-γ Induces IL-15 Trans-Presentation by Epithelial Cells via IRF1. J. Immunol. 2022, 208, 338–346. [Google Scholar] [CrossRef]
- Kim, Y.S.; Maslinski, W.; Zheng, X.X.; Stevens, A.C.; Li, X.C.; Tesch, G.H.; Kelley, V.R.; Strom, T.B. Targeting the IL-15 Receptor with an Antagonist IL-15 Mutant/Fcγ2a Protein Blocks Delayed-Type Hypersensitivity. J. Immunol. 1998, 160, 5742–5748. [Google Scholar]
- Rodríguez-Álvarez, Y.; Cabrales-Rico, A.; Diago-Abreu, D.; Correa-Arguelles, E.; Reyes-Acosta, O.; Puente-Pérez, P.; Pichardo-Díaz, D.; Urquiza-Noa, D.; Hernández-Santana, A.; Garay-Pérez, H.E. d -Amino acid substitutions and dimerization increase the biological activity and stability of an IL-15 antagonist peptide. J. Pept. Sci. 2021, 27, e3293. [Google Scholar] [CrossRef]
- Smadja, J.; Quéméner, A.; Maillasson, M.; Sicard, B.; Leray, A.; Arzel, L.; Lebreton, J.; Mortier, E.; Dubreuil, D.; Mathé-Allainmat, M. Rational modification, synthesis and biological evaluation of N-substituted phthalazinone derivatives designed to target interleukine-15 protein. Bioorganic Med. Chem. 2021, 39, 116161. [Google Scholar] [CrossRef]
- Wang, C.-W.; Yang, L.-Y.; Chen, C.-B.; Ho, H.-C.; Hung, S.-I.; Yang, C.-H.; Chang, C.-J.; Su, S.-C.; Hui, R.C.-Y.; Chin, S.-W.; et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J. Clin. Investig. 2018, 128, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, C.-W.; Chen, C.-B.; Wang, C.-W.; Chen, W.-T.; Cheng, B.; Ji, C.; Chung, W.-H. Evaluation of Combination Therapy with Etanercept and Systemic Corticosteroids for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Multicenter Observational Study. J. Allergy Clin. Immunol. Pract. 2022, 10, 1295–1304.e6. [Google Scholar] [CrossRef]
- Ouyang, S.; Hsuchou, H.; Kastin, A.J.; Pan, W. TNF Stimulates Nuclear Export and Secretion of IL-15 by Acting on CRM1 and ARF6. PLoS ONE 2013, 8, e69356. [Google Scholar] [CrossRef]
- Kageyama, Y.; Takahashi, M.; Torikai, E.; Suzuki, M.; Ichikawa, T.; Nagafusa, T.; Koide, Y.; Nagano, A. Treatment with anti-TNF-alpha antibody infliximab reduces serum IL-15 levels in patients with rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 505–509. [Google Scholar] [CrossRef]
- Ichikawa, T.; Kageyama, Y.; Kobayashi, H.; Kato, N.; Tsujimura, K.; Koide, Y. Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatol. Int. 2010, 30, 725–730. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellón, T.; González-Valle, O.; Sendagorta, E.; Lerma, V.; Martínez del Río, J.; Martínez, C.; Servera, G.; González-Herrada, C.; Cachafeiro, L.; Lorente, J.A.; et al. IL-15/IL-15Rα in SJS/TEN: Relevant Expression of IL15 and IL15RA in Affected Skin. Biomedicines 2022, 10, 1868. https://doi.org/10.3390/biomedicines10081868
Bellón T, González-Valle O, Sendagorta E, Lerma V, Martínez del Río J, Martínez C, Servera G, González-Herrada C, Cachafeiro L, Lorente JA, et al. IL-15/IL-15Rα in SJS/TEN: Relevant Expression of IL15 and IL15RA in Affected Skin. Biomedicines. 2022; 10(8):1868. https://doi.org/10.3390/biomedicines10081868
Chicago/Turabian StyleBellón, Teresa, Olga González-Valle, Elena Sendagorta, Victoria Lerma, Javier Martínez del Río, Celia Martínez, Guillermo Servera, Carlos González-Herrada, Lucía Cachafeiro, José A. Lorente, and et al. 2022. "IL-15/IL-15Rα in SJS/TEN: Relevant Expression of IL15 and IL15RA in Affected Skin" Biomedicines 10, no. 8: 1868. https://doi.org/10.3390/biomedicines10081868