The Upregulation of Caffeic Acid Phenethyl Ester on Growth Differentiation Factor 15 Inhibits Transforming Growth Factor β/Smad Signaling in Bladder Carcinoma Cells
Abstract
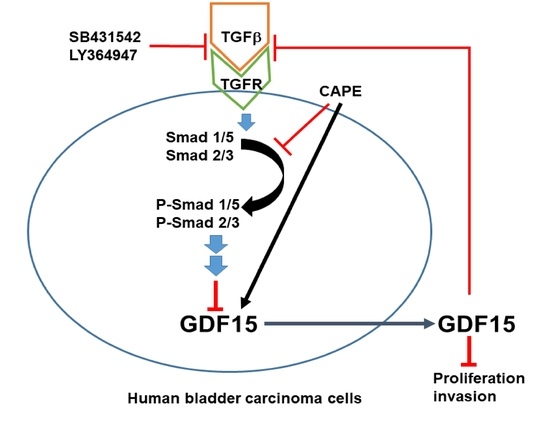
:1. Introduction
2. Materials and Methods
2.1. Cell lines and Cell Culture
2.2. Expression Vector Constructs and Stable Transfection
2.3. Immunoblot Assay
2.4. Real-Time Reverse Transcriptase-Polymerase Chain Reaction
2.5. Enzyme Linked Immunosorbent Assay
2.6. EdU Flow Cytometry Assay
2.7. WST-1 and CyQUANT Cell Proliferation Assay
2.8. Matrigel Invasion Assay
2.9. Reporter Vectors Construct, Transient Transfection, and Reporter Assay
2.10. Statistical Analysis
3. Results
3.1. Effect of TGFβ/Smad Signaling on the Expressions of GDF15, Maspin, and NDRG1 in Bladder Carcinoma Cells
3.2. Effect of TGFβ on the Expressions of GDF15, Maspin, and NDRG1 Is Blocked by Pretreatment of SB431542 in Bladder Carcinoma Cells
3.3. GDF15 Blocks the Effect of TGFβ/Smad Signaling on the Expressions of Maspin and NDRG1 in Bladder Carcinoma Cells
3.4. TGFβ Co-Treatment Blocks CAPE Inducing the Expressions of GDF15, Maspin, and NDRG1 in Bladder Carcinoma Cells
3.5. CAPE Treatment Acts as the Antagonist of TGFβ/Smad Signaling in Bladder Carcinoma Cells
3.6. The Activation of TGFβ on Cell Proliferation and Invasion Is Attenuated by CAPE Treatment in Bladder Carcinoma T24 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawicka, D.; Car, H.; Borawska, M.H.; Niklinski, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Yordanov, Y. Caffeic acid pheethyl ester (CAPE): Pharmacodynamics and potential for therapeutic application. Pharmacia 2019, 66, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumatz, A.; Asad, M.H.H.B. Caffeic acid phenethyl ester and therapeutic potentials. BioMed Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, L.C.; Chiang, K.C.; Feng, T.H.; Chang, K.S.; Chuang, S.T.; Chen, Y.J.; Tsui, K.H.; Lee, J.C.; Juang, H.H. Caffeic acid phenethyl ester upregulates N-myc downstream regulated gene 1 via ERK pathway to inhibit human oral cancer cell growth in vitro and in vivo. Mol. Nutr. Food Res. 2017, 61, 1600842. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yang, S.W.; Chang, K.P.; Feng, T.H.; Chang, K.S.; Tsui, K.H.; Shin, Y.S.; Chen, C.C.; Chao, M.; Juang, H.H. Caffeic acid phenethyl ester induces N-myc downstream regulated gene 1 to inhibit cell proliferation and invasion of human nasopharyngeal cancer cells. Int. J. Mol. Sci. 2018, 19, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.P.; Tsui, K.H.; Chang, K.S.; Sung, H.C.; Hsu, S.Y.; Lin, Y.H.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. Caffeic acid phenethyl ester inhibits the growth of bladder carcinoma cells by upregulating growth differentiation factor 15. Biomed. J. 2022, in press. [Google Scholar] [CrossRef]
- Chang, K.S.; Tsui, K.H.; Hsu, S.Y.; Sung, H.C.; Lin, Y.H.; Hou, C.P.; Yang, P.S.; Chen, C.L.; Feng, T.H.; Juang, H.H. The antitumor effect of caffeic acid phenethyl ester by downregulating mucosa-associated lymphoid tissue 1 via AR/p53/NF-κB signaling in prostate carcinoma cells. Cancers 2022, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Hébraud, B.; Bourin, H. Concise review: Growth differentiation factor 15 in pathology: A clinical role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef]
- Wang, Y.; Baek, S.J.; Eling, T.E. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem. Pharmacol. 2013, 85, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, P.J.; Djuffin, K.L.; Chintharlapallli, S.; Wu, X. GDF15 and growth control. Front. Physiol. 2018, 9, 1712. [Google Scholar] [CrossRef] [Green Version]
- Spanopoulou, A.; Gkretsi, V. Growth differentiation factor 15 (GDF15) in cancer cell metastasis: From the cells to the patients. Clin. Exp. Metastasis 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Costa, V.L.; Henrique, R.; Danielsen, S.A.; Duarte-Pereira, S.; Eknaes, M.; Skotheim, R.I.; Rodrigues, A.; Magalhães, J.S.; Oliveira, J.; Lothe, R.A.; et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin. Cancer Res. 2010, 16, 5842–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro-Reis, S.; Leça, L.; Almeida, M.; Antunes, L.; Monteiro, P.; Dias, P.C.; Morais, A.; Oliveira, J.; Henrique, R.; Jerónimo, C. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur. J. Cancer 2014, 50, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Hsu, S.Y.; Chung, L.C.; Lin, Y.H.; Feng, T.H.; Lee, T.Y.; Chang, P.L.; Juang, H.H. Growth differentiation factor-15: A p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci. Rep. 2015, 5, 12870. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.Q.; Xiong, G.Y.; Yang, K.W.; Zhang, L.; He, S.M.; Gong, Y.Q.; He, Q.; Li, X.Y.; Wang, Z.C.; Bao, Z.Q.; et al. Detection of urothelial carcinoma, upper tract urothelial carcinoma, bladder carcinoma, and urothelial carcinoma with gross hematuria using selected urine-DNA methylation biomarkers: A prospective, single-center study. Urol. Oncol. 2018, 36, 342.e15–342.e232. [Google Scholar] [CrossRef]
- Guan, B.; Xing, Y.; Xiong, G.; Cao, Z.; Fang, D.; Li, Y.; Zhan, Y.; Peng, D.; Liu, L.; Li, X.; et al. Predictive value of gene methylation for second recurrence following surgical treatment of first bladder recurrence of a primary upper-tract urothelial carcinoma. Oncol. Lett. 2018, 15, 9397–9405. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Xiong, G.; Fang, D.; Yang, X.; Li, X.; Zhou, L. Prognostic value of gene methylation and clinical factors in non-muscle-invasive upper tract urothelial carcinoma after radical nephroureterectomy. Clin. Genitourin. Cancer 2016, 14, e371–e378. [Google Scholar] [CrossRef]
- Prud’homme, G.J. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [Green Version]
- Izadifar, V.; De Boer, W.; Muscatelli-Groux, B.; Maille, P.; Van Der Kwast, T.H.; Chopin, D.K. Expression of transforming growth factor β1 and its receptors in normal human urothelium and human transitional cell carcinoma. Hum. Pathol. 1999, 50, 372–377. [Google Scholar] [CrossRef]
- Gonzalez, E.J.; Arms, L.; Vizzard, M.A. The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction. BioMed Res. Int. 2014, 2014, 120525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, T.; Li, X.; Yao, Y.; Othmane, B.; Chen, J.; Zu, X.; Ou, Z.; Hu, J. A novel TGF-β risk score predicts the clinical outcomes and tumour microenvironment phenotypes in bladder cancer. Front. Immunol. 2021, 12, 791924. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFβ in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, K.; Okamoto, M.; Oyasu, R. Transforming growth factor β type I receptor acts as a potent tumor suppressor in rat bladder carcinoma. Carcinogenesis 1997, 18, 1867–1870. [Google Scholar] [CrossRef]
- Champelovier, P.; El Atifi, M.; Mantel, F.; Rostaing, B.; Simon, A.; Berger, F.; Seigneurin, D. In vitro tumoral progression of human bladder carcinoma: Role for TGFβ. Euro. Urol. 2005, 48, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.T.; Wang, H.; Kingsley, E.A.; Risbridger, G.P.; Russell, P.J. Molecular profiling of bladder cancer: Involvement of the TGF-β pathway in bladder cancer progression. Cancer Lett. 2008, 265, 27–38. [Google Scholar] [CrossRef]
- Lang, K.; Kahveci, S.; Bonberg, N.; Wichert, K.; Behrens, T.; Hovanec, J.; Roghmann, F.; Noldus, J.; Tam, Y.C.; Tannapfel, A.; et al. TGFB1 protein is increased in the urine of patients with high-grade urothelial carcinomas, and promotes cell proliferation and migration. Int. J. Mol. Sci. 2019, 20, 4483. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, D.J.; Lyou, Y. Advances in immunotherapy and the TGF-β resistance pathway in metastatic bladder cancer. Cancers 2021, 13, 5724. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Liu, M.S.; Deng, T.B.; Wang, W.; Shao, W.Y.; Zhang, P. The TGF-β/Smad pathway inhibitor SB432542 enhances the antitumor effect of radiofrequency ablation on bladder cancer cells. OncoTargets Ther. 2019, 12, 7809–7821. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Sadoshima, J. GDF15, a cardioprotective TGF-beta superfamily protein. Circ. Res. 2006, 98, 294–297. [Google Scholar] [CrossRef] [Green Version]
- Artz, A.; Butz, S.; Vestweber, D. GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 2016, 128, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, M.; Koma, Y.I.; Kodama, T.; Nishio, M.; Shigeoka, M.; Yokozaki, H. Growth differentiation factor 15 promotes progression of esophageal squamous cells carcinoma via TGF-β II receptor activation. Pathobiology 2020, 87, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Strelau, J.; Bottner, M.; Lingor, P.; Suter-Crazzolara, C.; Galter, D.; Jaszai, J.; Sullivan, A.; Schober, A.; Krieglstein, K.; Unsicker, K. GDF15/MIC-1 a novel member of the TGF-β superfamily. J. Neural. Transm. Suppl. 2000, 60, 273–276. [Google Scholar]
- Muniyan, S.; Porthurajum, R.; Seshacharyulu, P. Macrophage inhibitory cytokine-1 in cancer: Beyond the cellular phenotype. Cancer Lett. 2022, 536, 215644. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Ned. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez1, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: Implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018, 28, 353–568. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Chen, S.; Zeng, J. TGFβ signaling: A complex role in tumorigenesis. Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, H.W.; Lee, S.S.; Chang, C.Y.; Lang, Y.D.; Jou, Y.S. A new switch for TGFβ in cancer. Cancer Res. 2019, 79, 3797–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Zhu, F.; Zhang, H.; Chen, D.; Zhang, X.; Gao, Q.; Li, Y. Conditional ablation of TGF-β signaling inhibits tumor progression and invasion in an induced mouse bladder cancer model. Sci. Rep. 2016, 6, 29479. [Google Scholar] [CrossRef] [PubMed]
- Stojnev, S.; Krstic, M.; Kokoris, J.C.; Conić, I.; Petković, I.; Ilić, S.; Milošević-Stevanović, J.; Veličković, L.J. Prognostic impact of canonical TGF-β signaling in urothelial bladder cancer. Medicina 2019, 55, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Huang, R.; Li, H.; Wang, B.; Chen, Y.; Chen, S.; Ou, K.; Wang, H. Secreted TGF-beta-induced protein promotes aggressive progression in bladder cancer cells. Cancer Manag. Res. 2019, 11, 6995–7006. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.C.; Randall, R.A.; Hill, C.S. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell. Biol. 2008, 28, 6889–6902. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Konigshoff, M.; Jayachandran, A.; Handley, D.; Seeger, W.; Kaminski, N.; Eickelberg, O. Transgelin is direct target of TGF-β/Smad3-dependent epithelial cell migration in lung fibrosis. FASEB J. 2008, 22, 1778–1789. [Google Scholar] [CrossRef]
- Cabezas, F.; Farfan, P.; Marzolo, M.P. The down regulation of megalin/LRP2 by transforming growth factor beta (TGF-β1) is mediated by SMAD2/3 signaling pathway. PLoS ONE 2019, 14, e0213127. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, E.; Tang, M.; Yang, X.; Wang, Y.; Quan, Z.; Wu, X.; Luo, C. The SMAD2/3 pathway is involved in hepaCAM-induced apoptosis by inhibiting the nuclear translocation of SMAD2/3 in bladder cancer cells. Tumor Biol. 2016, 37, 10731–10743. [Google Scholar] [CrossRef]
- Liu, G.L.; Liu, B.; Liu, T. GP73 promotes invasion and metastasis of bladder cancer by regulating the epithelial—Mesenchymal transition through the TGF-β1/Smad2 signalling pathway. J. Cell. Mol. Med. 2018, 22, 1650–1665. [Google Scholar]
- Zhang, N.; Bi, X.; Zeng, Y.; Zhu, Y.; Zhang, Z.; Liu, Y.; Wang, J.; Li, X.; Bi, J.; Kong, C. TGF-β1 promotes the migration and invasion of bladder carcinoma cells by increasing fascin1 expression. Oncol. Rep. 2016, 36, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hau, A.M.; Al-Ahmadie, H.A.; Harwalkar, J.; Shoskes, A.C.; Elson, P.; Beach, J.R.; Hussey, G.S.; Schiemann, W.P.; Egelhoff, T.T.; et al. Transforming growth factor-β is an upstream regulator of mammalian target of rpamycin complex 2-dependent bladder cancer cell migration and invasion. Am. J. Pathol. 2016, 186, 1351–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; He, S.; Zhan, Y.; He, A.; Fang, D.; Gong, Y.; Li, X.; Zhou, L. TGF-β induced transgelin promotes bladder cancer metastasis by regulating epithelial-mesencymal transition and invadopodia formation. EBioMedicine 2019, 47, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Jung, S.N.; Lim, M.A.; Oh, C.; Piao, Y.; Kim, J.H.; Liu, L.; Kang, Y.E.; Chang, J.W.; Won, H.R.; et al. Transcriptional regulation of GDF15 by EGR1 promotes head and neck cancer progression through a positive feedback loop. Int. J. Mol. Sci. 2021, 22, 11151. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.T.; Kuo, Y.H.; Su, M.J. Antifibrotic effects of KS370G, a caffeamide derivative, in renal ischemial-reperfusion injured mice and renal tubular epithelial cells. Sci. Rep. 2014, 4, 5814. [Google Scholar] [CrossRef] [Green Version]
- Omene, C.; Ma, L.; Moore, J.; Ouyang, H.; Illa-Bochaca, I.; Chou, W.; Patel, M.S.; Sebastiano, C.; Demaria, S.; Mao, J.H.; et al. Aggressive mammary cancers lacking lymphocytic infiltration arise in irradiated mice and can be prevented by dietary intervention. Cancer Immunol. Res. 2020, 8, 217–229. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.-P.; Tsui, K.-H.; Chen, S.-T.; Chang, K.-S.; Sung, H.-C.; Hsu, S.-Y.; Lin, Y.-H.; Feng, T.-H.; Juang, H.-H. The Upregulation of Caffeic Acid Phenethyl Ester on Growth Differentiation Factor 15 Inhibits Transforming Growth Factor β/Smad Signaling in Bladder Carcinoma Cells. Biomedicines 2022, 10, 1625. https://doi.org/10.3390/biomedicines10071625
Hou C-P, Tsui K-H, Chen S-T, Chang K-S, Sung H-C, Hsu S-Y, Lin Y-H, Feng T-H, Juang H-H. The Upregulation of Caffeic Acid Phenethyl Ester on Growth Differentiation Factor 15 Inhibits Transforming Growth Factor β/Smad Signaling in Bladder Carcinoma Cells. Biomedicines. 2022; 10(7):1625. https://doi.org/10.3390/biomedicines10071625
Chicago/Turabian StyleHou, Chen-Pang, Ke-Hung Tsui, Syue-Ting Chen, Kang-Shuo Chang, Hsin-Ching Sung, Shu-Yuan Hsu, Yu-Hsiang Lin, Tsui-Hsia Feng, and Horng-Heng Juang. 2022. "The Upregulation of Caffeic Acid Phenethyl Ester on Growth Differentiation Factor 15 Inhibits Transforming Growth Factor β/Smad Signaling in Bladder Carcinoma Cells" Biomedicines 10, no. 7: 1625. https://doi.org/10.3390/biomedicines10071625