Saxagliptin Cardiotoxicity in Chronic Heart Failure: The Role of DPP4 in the Regulation of Neuropeptide Tone
Abstract
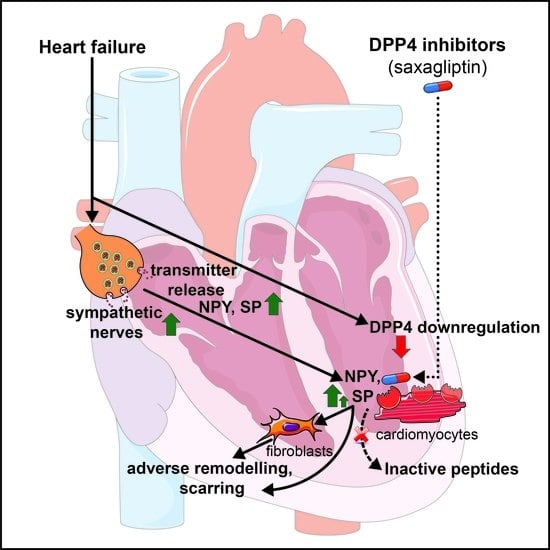
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viability Measurements
2.3. Co-Culture of Neonatal Rat Cardiac Fibroblasts and Myocytes
2.4. Scratch Assay
2.5. Human Heart Tissue Collection
2.6. RNA Scope® In Situ Hybridization
2.7. DPP4 Protein Expression in the Heart
2.8. SP-like Immunoreactivity
2.9. NPY-like Immunoreactivity
2.10. Statistical Analysis
3. Results
3.1. Protein Expression of DPP4 and NPY Decreased in Human Failing Heart Samples
3.2. DPP4 mRNA Is Primarily Localized in Cardiomyocytes of the Human Left Ventricle
3.3. DPP4 Inhibition and Neuropeptide Substrates Do Not Affect Cell Viability
3.4. Both Saxagliptin and the Neuropeptides Alter the Migration Capacity of Cardiac Fibroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CD68 | cluster of differentiation 68 |
| CON | control |
| Cy3 | cyanine 3 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCM | dilated cardiomyopathy |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | dimethyl sulfoxide |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| DPP4 | dipeptidyl-peptidase-4 |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HBSS | Hank’s balanced salt solution |
| HRP | horseradish peroxidase |
| ICM | ischemic cardiomyopathy |
| mRNA | messenger RNA |
| PECAM-1 | platelet endothelial cell adhesion molecule 1 |
| RFU | relative fluorescent unit |
| RYR2 | ryanodine receptor 2 |
| SEM | standard error of the mean |
| VIM | vimentin |
References
- Braunwald, E. Heart failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, C.J.; Franco-Cereceda, A.; Saria, A.; Lundberg, J.M.; Theodorsson-Norheim, E.; Hökfelt, T. Distribution and origin of substance P- and neuropeptide Y-immunoreactive nerves in the guinea-pig heart. Cell Tissue Res. 1986, 243, 477–485. [Google Scholar] [CrossRef]
- Papka, R.E.; Urban, L. Distribution, origin and sensitivity to capsaicin of primary afferent substance P-immunoreactive nerves in the heart. Acta Physiol. Hung. 1987, 69, 459–468. [Google Scholar] [PubMed]
- Wharton, J.; Polak, J.M.; McGregor, G.P.; Bishop, A.E.; Bloom, S.R. The distribution of substrate P-like immunoreactive nerves in the guinea-pig heart. Neuroscience 1981, 6, 2193–2204. [Google Scholar] [CrossRef]
- Rysevaite, K.; Saburkina, I.; Pauziene, N.; Vaitkevicius, R.; Noujaim, S.F.; Jalife, J.; Pauza, D.H. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 2011, 8, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Milner, P.; Ralevic, V.; Hopwood, A.M.; Fehér, E.; Lincoln, J.; Kirkpatrick, K.A.; Burnstock, G. Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia 1989, 45, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Pozsgai, G.; Hajna, Z.; Helyes, Z.; Szolcsányi, J. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br. J. Clin. Pharmacol. 2014, 77, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Mak, I.T.; Chmielinska, J.J.; Kramer, J.H.; Spurney, C.F.; Weglicki, W.B. Loss of neutral endopeptidase activity contributes to neutrophil activation and cardiac dysfunction during chronic hypomagnesemia: Protection by substance P receptor blockade. Exp. Clin. Cardiol. 2011, 16, 121–124. [Google Scholar]
- Weglicki, W.B.; Phillips, T.M. Pathobiology of magnesium deficiency: A cytokine/neurogenic inflammation hypothesis. Am. J. Physiol. 1992, 263, R734–R737. [Google Scholar] [CrossRef] [Green Version]
- Weglicki, W.B.; Mak, I.T.; Phillips, T.M. Blockade of cardiac inflammation in Mg2+ deficiency by substance P receptor inhibition. Circ. Res. 1994, 74, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Dehlin, H.M.; Manteufel, E.J.; Monroe, A.L.; Reimer, M.H., Jr.; Levick, S.P. Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int. J. Cardiol. 2013, 168, 4643–4651. [Google Scholar] [CrossRef] [Green Version]
- Kumaran, C.; Shivakumar, K. Calcium- and superoxide anion-mediated mitogenic action of substance P on cardiac fibroblasts. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H1855–H1862. [Google Scholar] [CrossRef] [Green Version]
- Callanan, E.Y.; Lee, E.W.; Tilan, J.U.; Winaver, J.; Haramati, A.; Mulroney, S.E.; Zukowska, Z. Renal and cardiac neuropeptide Y and NPY receptors in a rat model of congestive heart failure. Am. J. Physiol. Ren. Physiol. 2007, 293, F1811–F1817. [Google Scholar] [CrossRef]
- McDermott, B.J.; Bell, D. NPY and cardiac diseases. Curr. Top. Med. Chem. 2007, 7, 1692–1703. [Google Scholar] [CrossRef]
- Ullman, B.; Hulting, J.; Lundberg, J.M. Prognostic value of plasma neuropeptide-Y in coronary care unit patients with and without acute myocardial infarction. Eur. Heart J. 1994, 15, 454–461. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Bohm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Gallwitz, B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 479–486. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Ahrén, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef]
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef]
- Zhu, L.; Tamvakopoulos, C.; Xie, D.; Dragovic, J.; Shen, X.; Fenyk-Melody, J.E.; Schmidt, K.; Bagchi, A.; Griffin, P.R.; Thornberry, N.A.; et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38). J. Biol. Chem. 2003, 278, 22418–22423. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.R.; Drucker, D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012, 33, 187–215. [Google Scholar] [CrossRef]
- Nadasdi, A.; Sinkovits, G.; Bobek, I.; Lakatos, B.; Forhecz, Z.; Prohaszka, Z.Z.; Reti, M.; Arato, M.; Cseh, G.; Masszi, T.; et al. Decreased circulating dipeptidyl peptidase-4 enzyme activity is prognostic for severe outcomes in COVID-19 inpatients. Biomark. Med. 2022, 16, 317–330. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Makkos, A.; Szantai, A.; Paloczi, J.; Pipis, J.; Kiss, B.; Poggi, P.; Ferdinandy, P.; Chatgilialoglu, A.; Gorbe, A. A Comorbidity Model of Myocardial Ischemia/Reperfusion Injury and Hypercholesterolemia in Rat Cardiac Myocyte Cultures. Front. Physiol. 2019, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Brenner, G.B.; Makkos, A.; Nagy, C.T.; Onodi, Z.; Sayour, N.V.; Gergely, T.G.; Kiss, B.; Gorbe, A.; Saghy, E.; Zadori, Z.S.; et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells 2020, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Jelemensky, M.; Kovacshazi, C.; Ferenczyova, K.; Hofbauerova, M.; Kiss, B.; Pallinger, E.; Kittel, A.; Sayour, V.N.; Gorbe, A.; Pelyhe, C.; et al. Helium Conditioning Increases Cardiac Fibroblast Migration Which Effect Is Not Propagated via Soluble Factors or Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 10504. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Pipicz, M.; Baan, J.A.; Baranyai, T.; Koncsos, G.; Leszek, P.; Kusmierczyk, M.; Sanchez-Cabo, F.; Garcia-Pavia, P.; Brenner, G.J.; et al. Alternative Splicing of NOX4 in the Failing Human Heart. Front. Physiol. 2017, 8, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voros, I.; Saghy, E.; Pohoczky, K.; Makkos, A.; Onodi, Z.; Brenner, G.B.; Baranyai, T.; Agg, B.; Varadi, B.; Kemeny, A.; et al. Somatostatin and Its Receptors in Myocardial Ischemia/Reperfusion Injury and Cardioprotection. Front. Pharmacol. 2021, 12, 663655. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Nagy, J.A.; Pyne, K.; Dvorak, H.F.; Dvorak, A.M. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J. Histochem. Cytochem. 2004, 52, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Lawson, J.S.; Syme, H.M.; Wheeler-Jones, C.P.D.; Elliott, J. Characterisation of feline renal cortical fibroblast cultures and their transcriptional response to transforming growth factor beta1. BMC Vet. Res. 2018, 14, 76. [Google Scholar] [CrossRef]
- Kim, H.D. Expression of intermediate filament desmin and vimentin in the human fetal heart. Anat. Rec. Off. Publ. Am. Assoc. Anat. 1996, 246, 271–278. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Macrophage-specific gene expression: Current paradigms and future challenges. Int. J. Hematol. 2002, 76, 6–15. [Google Scholar] [CrossRef]
- Nemeth, J.; Helyes, Z.; Gorcs, T.; Gardi, J.; Pinter, E.; Szolcsanyi, J. Development of somatostatin radioimmunoassay for the measurement of plasma and tissue contents of hormone. Acta Physiol. Hung. 1996, 84, 313–315. [Google Scholar]
- Wang, J.; Hao, D.; Zeng, L.; Zhang, Q.; Huang, W. Neuropeptide Y mediates cardiac hypertrophy through microRNA-216b/FoxO4 signaling pathway. Int. J. Med. Sci. 2021, 18, 18–28. [Google Scholar] [CrossRef]
- Dehlin, H.M.; Levick, S.P. Substance P in heart failure: The good and the bad. Int. J. Cardiol. 2014, 170, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.M.J.; Green, P.; Tapoulal, N.; Lewandowski, A.J.; Leeson, P.; Herring, N. The Role of Neuropeptide Y in Cardiovascular Health and Disease. Front. Physiol. 2018, 9, 1281. [Google Scholar] [CrossRef]
- Widiapradja, A.; Chunduri, P.; Levick, S.P. The role of neuropeptides in adverse myocardial remodeling and heart failure. Cell Mol. Life Sci. 2017, 74, 2019–2038. [Google Scholar] [CrossRef]
- Costoli, T.; Sgoifo, A.; Stilli, D.; Flugge, G.; Adriani, W.; Laviola, G.; Fuchs, E.; Pedrazzini, T.; Musso, E. Behavioural, neural and cardiovascular adaptations in mice lacking the NPY Y1 receptor. Neurosci. Biobehav. Rev. 2005, 29, 113–123. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Baczkó, I.; Bencsik, P.; Giricz, Z.; Görbe, A.; Pacher, P.; Varga, Z.V.; Varró, A.; Schulz, R. Definition of hidden drug cardiotoxicity: Paradigm change in cardiac safety testing and its clinical implications. Eur. Heart J. 2019, 40, 1771–1777. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F.; Kubant, R.; Walter, M.F.; Bellamine, A.; Jacoby, A.; Mizuno, Y.; Malinski, T. Effect of enhanced glycemic control with saxagliptin on endothelial nitric oxide release and CD40 levels in obese rats. J. Atheroscler. Thromb. 2011, 18, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Ta, N.N.; Schuyler, C.A.; Li, Y.; Lopes-Virella, M.F.; Huang, Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2011, 58, 157–166. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Chatterjee, N.A.; Gonzales, M.J.; Gornbein, J.; Liu, K.; Li, D.; Paterson, D.J.; Shivkumar, K.; Singh, J.P.; Herring, N. Coronary Sinus Neuropeptide Y Levels and Adverse Outcomes in Patients With Stable Chronic Heart Failure. JAMA Cardiol. 2020, 5, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Rao, X.; Rajagopalan, S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis 2013, 226, 305–314. [Google Scholar] [CrossRef]
- Gorrell, M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005, 108, 277–292. [Google Scholar] [CrossRef]
- Heike, M.; Mobius, U.; Knuth, A.; Meuer, S.; Meyer zum Buschenfelde, K.H. Tissue distribution of the T cell activation antigen Ta1. Serological, immunohistochemical and biochemical investigations. Clin. Exp. Immunol. 1988, 74, 431–434. [Google Scholar]
- Dinjens, W.N.; ten Kate, J.; van der Linden, E.P.; Wijnen, J.T.; Khan, P.M.; Bosman, F.T. Distribution of adenosine deaminase complexing protein (ADCP) in human tissues. J. Histochem. Cytochem. 1989, 37, 1869–1875. [Google Scholar] [CrossRef] [Green Version]
- Shigeta, T.; Aoyama, M.; Bando, Y.K.; Monji, A.; Mitsui, T.; Takatsu, M.; Cheng, X.W.; Okumura, T.; Hirashiki, A.; Nagata, K.; et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation 2012, 126, 1838–1851. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, P.M.; Coats, A.J.S.; Ponikowski, P.; Filippatos, G.; Huelsmann, M.; Jhund, P.S.; Polovina, M.M.; Komajda, M.; Seferovic, J.; Sari, I.; et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. Eur. J. Heart Fail. 2020, 22, 196–213. [Google Scholar] [CrossRef] [Green Version]
- Kongwatcharapong, J.; Dilokthornsakul, P.; Nathisuwan, S.; Phrommintikul, A.; Chaiyakunapruk, N. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: A meta-analysis of randomized clinical trials. Int. J. Cardiol. 2016, 211, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.A.; Mosenzon, O.; Im, K.; Umez-Eronini, A.A.; et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Goud, A.; D’Souza, J.; Dahagam, C.; Rao, X.; Rajagopalan, S.; Zhong, J. DPP4 inhibitors and cardiovascular outcomes: Safety on heart failure. Heart Fail. Rev. 2017, 22, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Parker, A.; Frederich, R.; Donovan, M.; Hirshberg, B. Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: Pooled analysis of 20 clinical trials. Cardiovasc. Diabetol. 2014, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Men, P.; Li, X.T.; Tang, H.L.; Zhai, S.D. Efficacy and safety of saxagliptin in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0197321. [Google Scholar] [CrossRef]
- Pollack, P.S.; Chadwick, K.D.; Smith, D.M.; Billger, M.; Hirshberg, B.; Iqbal, N.; Boulton, D.W. Nonclinical and clinical pharmacology evidence for cardiovascular safety of saxagliptin. Cardiovasc. Diabetol. 2017, 16, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleenor, B.S.; Ouyang, A.; Olver, T.D.; Hiemstra, J.A.; Cobb, M.S.; Minervini, G.; Emter, C.A. Saxagliptin Prevents Increased Coronary Vascular Stiffness in Aortic-Banded Mini Swine. Hypertension 2018, 72, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.; Kimoto, N.; Kitayama, T.; Kunori, S. Cardiac DPP-4 inhibition by saxagliptin ameliorates isoproterenol-induced myocardial remodeling and cardiac diastolic dysfunction in rats. J. Pharmacol. Sci. 2016, 132, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradic, J.; Milosavljevic, I.; Bolevich, S.; Litvitskiy, P.F.; Jeremic, N.; Bolevich, S.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Jovicic, N.; et al. Dipeptidyl peptidase 4 inhibitors attenuate cardiac ischaemia-reperfusion injury in rats with diabetes mellitus type 2. Clin. Exp. Pharmacol. Physiol. 2021, 48, 575–584. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Chen, Z.; Yao, Q. DPP-4 inhibitor saxagliptin ameliorates oxygen deprivation/reoxygenation-induced brain endothelial injury. Am. J. Transl. Res. 2019, 11, 6316–6325. [Google Scholar]
- Keller, A.C.; Knaub, L.A.; Miller, M.W.; Birdsey, N.; Klemm, D.J.; Reusch, J.E. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J. Cardiovasc. Pharmacol. 2015, 65, 137–147. [Google Scholar] [CrossRef]
- Esposito, G.; Cappetta, D.; Russo, R.; Rivellino, A.; Ciuffreda, L.P.; Roviezzo, F.; Piegari, E.; Berrino, L.; Rossi, F.; De Angelis, A.; et al. Sitagliptin reduces inflammation, fibrosis and preserves diastolic function in a rat model of heart failure with preserved ejection fraction. Br. J. Pharmacol. 2017, 174, 4070–4086. [Google Scholar] [CrossRef] [Green Version]
- Ghersi, G.; Chen, W.; Lee, E.W.; Zukowska, Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides 2001, 22, 453–458. [Google Scholar] [CrossRef]
- Lee, E.W.; Michalkiewicz, M.; Kitlinska, J.; Kalezic, I.; Switalska, H.; Yoo, P.; Sangkharat, A.; Ji, H.; Li, L.; Michalkiewicz, T.; et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J. Clin. Investig. 2003, 111, 1853–1862. [Google Scholar] [CrossRef] [Green Version]
- Abualsaud, N.; Caprio, L.; Galli, S.; Krawczyk, E.; Alamri, L.; Zhu, S.; Gallicano, G.I.; Kitlinska, J. Neuropeptide Y/Y5 Receptor Pathway Stimulates Neuroblastoma Cell Motility Through RhoA Activation. Front. Cell Dev. Biol. 2020, 8, 627090. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vörös, I.; Onódi, Z.; Tóth, V.É.; Gergely, T.G.; Sághy, É.; Görbe, A.; Kemény, Á.; Leszek, P.; Helyes, Z.; Ferdinandy, P.; et al. Saxagliptin Cardiotoxicity in Chronic Heart Failure: The Role of DPP4 in the Regulation of Neuropeptide Tone. Biomedicines 2022, 10, 1573. https://doi.org/10.3390/biomedicines10071573
Vörös I, Onódi Z, Tóth VÉ, Gergely TG, Sághy É, Görbe A, Kemény Á, Leszek P, Helyes Z, Ferdinandy P, et al. Saxagliptin Cardiotoxicity in Chronic Heart Failure: The Role of DPP4 in the Regulation of Neuropeptide Tone. Biomedicines. 2022; 10(7):1573. https://doi.org/10.3390/biomedicines10071573
Chicago/Turabian StyleVörös, Imre, Zsófia Onódi, Viktória Éva Tóth, Tamás G. Gergely, Éva Sághy, Anikó Görbe, Ágnes Kemény, Przemyslaw Leszek, Zsuzsanna Helyes, Péter Ferdinandy, and et al. 2022. "Saxagliptin Cardiotoxicity in Chronic Heart Failure: The Role of DPP4 in the Regulation of Neuropeptide Tone" Biomedicines 10, no. 7: 1573. https://doi.org/10.3390/biomedicines10071573