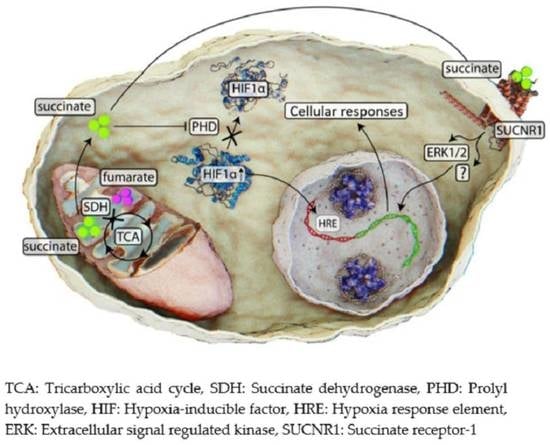
Succinate at the Crossroad of Metabolism and Angiogenesis: Roles of SDH, HIF1α and SUCNR1
Abstract
:1. Introduction
2. Angiogenesis
3. Metabolic Regulation of Angiogenesis
4. Succinate in Biological Fluids and Tissues between Health and Disease
5. SDH Alterations as a Cause of Succinate Accumulation
6. Succinate Accumulation and Induction of Pseudohypoxia
7. Succinate Signaling via SUCNR1
8. Succinate as a Regulator of Angiogenesis
| Tissue/Cells | Pathological Context | Mechanism/Effectors | References |
|---|---|---|---|
| Neuroendocrine | Pheochromocytoma paraganglioma | SDH mutation | [136,137,138] |
| Breast | Triple-negative breast cancer | HIF1α stabilization | [143] |
| Melanoma cell line | Melanoma | HIF1α stabilization | [140] |
| Intestine | Gastric cancer | SUCNR1-mediated ERK1/2 and STAT3 phosphorylation | [132] |
| Colorectal cancer | HIF1α stabilization | [139] | |
| Oral cavity, pharynx and larynx | Head and neck squamous cell carcinoma | Increased expression of SUCNR1, HIF1α, SDHA and SDHB | [34] |
| Placenta | Gestational diabetes | SUCNR1-mediated ERK1/2 phosphorylation | [42] |
| Retina/retinal ganglion cells | Diabetic retinopathy | SUCNR1-mediated ERK1/2/COX-2 signaling | [55] |
| Proliferative ischemic retinopathy | SUCNR1 activation | [144,145] | |
| Synovium | Rheumatoid arthritis | HIF1α induction and via SUCNR1 | [60] |
| Peripheral limb muscles | Acute peripheral ischemia | Increased SUCNR1 expression | [146] |
| Brain | Hypoxia/ischemia brain injury | SUCNR1 regulation of prostaglandin E2–prostaglandin E receptor 4 | [147] |
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otrock, Z.K.; Mahfouz, R.A.R.; Makarem, J.A.; Shamseddine, A.I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K. Angiogenesis: A curse or cure? Postgrad. Med. J. 2005, 81, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Duchen, M.R. Endothelial Mitochondria. Circ. Res. 2007, 100, 1128–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dranka, B.P.; Hill, B.G.; Darley-Usmar, V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010, 48, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, M.; Shepherd, B.R.; Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Pan, Y.; Acevedo, L.M.; Shadel, G.S.; Sessa, W.C. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J. Cell Biol. 2008, 180, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Al-Mehdi, A.-B.; Pastukh, V.M.; Swiger, B.M.; Reed, D.J.; Patel, M.R.; Bardwell, G.C.; Pastukh, V.V.; Alexeyev, M.F.; Gillespie, M.N. Perinuclear Mitochondrial Clustering Creates an Oxidant-Rich Nuclear Domain Required for Hypoxia-Induced Transcription. Sci. Signal. 2012, 5, ra47. [Google Scholar] [CrossRef] [Green Version]
- Coutelle, O.; Hornig-Do, H.; Witt, A.; Andree, M.; Schiffmann, L.M.; Piekarek, M.; Brinkmann, K.; Seeger, J.M.; Liwschitz, M.; Miwa, S.; et al. Embelin inhibits endothelial mitochondrial respiration and impairs neoangiogenesis during tumor growth and wound healing. EMBO Mol. Med. 2014, 6, 624–639. [Google Scholar] [CrossRef]
- Lapel, M.; Weston, P.; Strassheim, D.; Karoor, V.; Burns, N.; Lyubchenko, T.; Paucek, P.; Stenmark, K.R.; Gerasimovskaya, E.V. Glycolysis and oxidative phosphorylation are essential for purinergic receptor-mediated angiogenic responses in vasa vasorum endothelial cells. Am. J. Physiol. Physiol. 2017, 312, C56–C70. [Google Scholar] [CrossRef] [Green Version]
- Pozza, E.D.; Dando, I.; Pacchiana, R.; Liboi, E.; Scupoli, M.T.; Donadelli, M.; Palmieri, M. Regulation of succinate dehydrogenase and role of succinate in cancer. Semin. Cell Dev. Biol. 2020, 98, 4–14. [Google Scholar] [CrossRef]
- Bandara, A.B.; Drake, J.C.; Brown, D.A. Complex II subunit SDHD is critical for cell growth and metabolism, which can be partially restored with a synthetic ubiquinone analog. BMC Mol. Cell Biol. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraisl, P.; Mazzone, M.; Schmidt, T.; Carmeliet, P. Regulation of Angiogenesis by Oxygen and Metabolism. Dev. Cell 2009, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bierhansl, L.; Conradi, L.-C.; Treps, L.; Dewerchin, M.; Carmeliet, P. Central Role of Metabolism in Endothelial Cell Function and Vascular Disease. Physiology 2017, 32, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eelen, G.; De Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Dallinga, M.G.; Boas, S.E.; Klaassen, I.; Merks, R.H.; van Noorden, C.J.; Schlingemann, R.O. Tip Cells in Angiogenesis. eLS 2015, 1–10. [Google Scholar] [CrossRef]
- Makanya, A.N.; Hlushchuk, R.; Djonov, V.G. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 2009, 12, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Salven, P. Stem cells in tumor angiogenesis. J. Mol. Cell. Cardiol. 2011, 50, 290–295. [Google Scholar] [CrossRef]
- Zimta, A.-A.; Baru, O.; Badea, M.; Buduru, S.D.; Berindan-Neagoe, I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. Int. J. Mol. Sci. 2019, 20, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Qian, B.-Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.; Clemons, M.; et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res 2015, 75, 3479–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flournoy, J.; Ashkanani, S.; Chen, Y. Mechanical regulation of signal transduction in angiogenesis. Front. Cell Dev. Biol. 2022, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Valerio, G.; Casanovas, O. Angiogenesis and Metabolism: Entwined for Therapy Resistance. Trends Cancer 2016, 3, 10–18. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Du, W.; Ren, L.; Hamblin, M.; Fan, Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.S.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty Acid Carbon Is Essential for Dntp Synthesis in Endothelial Cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; O’Neill, L.A. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell 2018, 174, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Sadagopan, N.; Li, W.; Roberds, S.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating Succinate is Elevated in Rodent Models of Hypertension and Metabolic Disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar] [CrossRef]
- Kushnir, M.M.; Komaromy-Hiller, G.; Shushan, B.; Urry, F.M.; Roberts, W.L. Analysis of Dicarboxylic Acids by Tandem Mass Spectrometry. High-Throughput Quantitative Measurement of Methylmalonic Acid in Serum, Plasma, and Urine. Clin. Chem. 2001, 47, 1993–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, C.J.; Rocha-Franco, J.A.; A Sousa, P.; Santos, A.K.; Ladeira, M.; Rocha-Resende, C.; Ladeira, L.O.; Resende, R.R.; Botoni, F.A.; Melo, M.B.; et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun. Signal. 2014, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Terra, X.; Ceperuelo-Mallafré, V.; Merma, C.; Benaiges, E.; Bosch, R.; Castillo, P.; Flores, J.; León, X.; Valduvieco, I.; Basté, N.; et al. Succinate Pathway in Head and Neck Squamous Cell Carcinoma: Potential as a Diagnostic and Prognostic Marker. Cancers 2021, 13, 1653. [Google Scholar] [CrossRef] [PubMed]
- Hobert, J.A.; Mester, J.L.; Moline, J.; Eng, C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation–positive individuals. Genet. Med. 2012, 14, 616–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Ortiz-Alvarez, L.; Di, X.; Jurado-Fasoli, L.; Xu, H.; Ceperuelo-Mallafré, V.; Núñez-Roa, C.; Kohler, I.; Segura-Carretero, A.; et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 2021, 20, 151. [Google Scholar] [CrossRef]
- Zhu, J.; Djukovic, D.; Deng, L.; Gu, H.; Himmati, F.; Abu Zaid, M.; Chiorean, E.G.; Raftery, D. Targeted serum metabolite profiling and sequential metabolite ratio analysis for colorectal cancer progression monitoring. Anal. Bioanal. Chem. 2015, 407, 7857–7863. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.-G.; Zhao, W.; Zhang, J.; Wu, X.; Hu, J.; Yin, G.-C.; Xu, Y.-J. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget 2017, 8, 63890–63900. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Ortiz-Masia, M.D.; Salvador, P.; Gisbert-Ferrándiz, L.; Hernandez, C.; Hausmann, M.; Rogler, G.; Esplugues, J.V.; Hinojosa, J.; Alós, R.; et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2018, 12, 178–187. [Google Scholar] [CrossRef]
- Slaughter, A.L.; D’Alessandro, A.; Moore, E.E.; Banerjee, A.; Silliman, C.C.; Hansen, K.C.; Reisz, J.A.; Fragoso, M.; Wither, M.J.; Bacon, A.W.; et al. Glutamine metabolism drives succinate accumulation in plasma and the lung during hemorrhagic shock. J. Trauma Acute Care Surg. 2016, 81, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atallah, R.; Gindlhuber, J.; Platzer, W.; Bärnthaler, T.; Tatzl, E.; Toller, W.; Strutz, J.; Rittchen, S.; Luschnig, P.; Birner-Gruenberger, R.; et al. SUCNR1 Is Expressed in Human Placenta and Mediates Angiogenesis: Significance in Gestational Diabetes. Int. J. Mol. Sci. 2021, 22, 12048. [Google Scholar] [CrossRef] [PubMed]
- Tessem, M.-B.; Bertilsson, H.; Angelsen, A.; Bathen, T.F.; Drabløs, F.; Rye, M.B. A Balanced Tissue Composition Reveals New Metabolic and Gene Expression Markers in Prostate Cancer. PLoS ONE 2016, 11, e0153727. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Klocker, H.; Oberacher, H.; Gnaiger, E.; Neuwirt, H.; Sampson, N.; Eder, I.E. Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Q.; Wang, C.; Li, B. Metabolomic Analysis Using Liquid Chromatography/Mass Spectrometry for Gastric Cancer. Appl. Biochem. Biotechnol. 2015, 176, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cai, G.; Su, M.; Chen, T.; Liu, Y.; Xu, Y.; Ni, Y.; Zhao, A.; Cai, S.; Xu, L.X.; et al. Urinary Metabonomic Study on Colorectal Cancer. J. Proteome Res. 2010, 9, 1627–1634. [Google Scholar] [CrossRef]
- Davis, V.W.; Schiller, D.E.; Eurich, D.; Sawyer, M.B. Urinary metabolomic signature of esophageal cancer and Barrett’s esophagus. World J. Surg. Oncol. 2012, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Nishiumi, S.; Suzuki, M.; Kobayashi, T.; Matsubara, A.; Azuma, T.; Yoshida, M. Metabolomics for Biomarker Discovery in Gastroenterological Cancer. Metabolites 2014, 4, 547–571. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, B.M.; Stirdivant, S.M.; Mitchell, M.W.; Wulff, J.E.; McDunn, J.E.; Li, Z.; Dennis-Barrie, A.; Neri, B.P.; Milburn, M.V.; Lotan, Y.; et al. Bladder Cancer Biomarker Discovery Using Global Metabolomic Profiling of Urine. PLoS ONE 2014, 9, e115870. [Google Scholar] [CrossRef] [Green Version]
- Cala, M.; Aldana, J.; Sánchez, J.; Guio, J.; Meesters, R.J. Urinary metabolite and lipid alterations in Colombian Hispanic women with breast cancer: A pilot study. J. Pharm. Biomed. Anal. 2018, 152, 234–241. [Google Scholar] [CrossRef]
- Matsumoto, M.; Suzuma, K.; Maki, T.; Kinoshita, H.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Succinate Increases in the Vitreous Fluid of Patients With Active Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2012, 153, 896–902.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014, 4, 6802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454–29464. [Google Scholar] [CrossRef] [PubMed]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Hu, J.; Du, S.; Chen, Y.; Wang, S.; Wu, Q. ERK1/2/COX-2/PGE2 signaling pathway mediates gpr91-dependent VEGF release in streptozotocin-induced diabetes. Mol. Vis. 2014, 20, 1109–1121. [Google Scholar]
- Wang, Y.; Zhang, X.; Yao, H.; Chen, X.; Shang, L.; Li, P.; Cui, X.; Zeng, J. Peroxisome-generated succinate induces lipid accumulation and oxidative stress in the kidneys of diabetic mice. J. Biol. Chem. 2022, 298, 101660. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Sokolov, A.P.; Kondrashova, M.N. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free Radic. Biol. Med. 2006, 41, 56–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2019, 28, 101365. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, Y.; Wang, C.; Xia, W.-R.; Zheng, J.-Y.; Yang, J.; Liu, B.; Liu, J.-Q.; Liu, L.-F. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free. Radic. Biol. Med. 2018, 126, 1–14. [Google Scholar] [CrossRef]
- Guo, Y.; Cho, S.W.; Saxena, D.; Li, X. Multifaceted Actions of Succinate as a Signaling Transmitter Vary with Its Cellular Locations. Endocrinol. Metab. 2020, 35, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Dawe, N.; Van Limbergen, J. The Role of Succinate in the Regulation of Intestinal Inflammation. Nutrients 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Leite, A.Z.; Rodrigues, N.D.C.; Gonzaga, M.I.; Paiolo, J.C.C.; De Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Jr, E.M.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, G.; Yehezkel, D.; Hoffman, D.; Mattioli, C.C.; Fremder, M.; Ben-Arosh, H.; Vainman, L.; Nissani, N.; Hen-Avivi, S.; Brenner, S.; et al. Host succinate is an activation signal for Salmonella virulence during intracellular infection. Science 2021, 371, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.E.; Brown, A.K.; Taus, L.; Khoury, J.; Glover, F.; Kami, K.; Sarangarajan, R.; Walshe, T.E.; Narain, N.R.; Kiebish, M.A.; et al. Mycoplasma infection and hypoxia initiate succinate accumulation and release in the VM-M3 cancer cells. Biochim. Biophys. Acta 2018, 1859, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2017, 72, 106–116. [Google Scholar] [CrossRef]
- Kim, E.; Rath, E.; Tsang, V.H.M.; Duff, A.; Robinson, B.G.; Church, W.; Benn, D.E.; Dwight, T.; Clifton-Bligh, R. Structural and functional consequences of succinate dehydrogenase subunit B mutations. Endocr.-Relat. Cancer 2015, 22, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta Bioenergy 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Klink, B.; Nacke, B.; De Cubas, A.A.; Mangelis, A.; Rapizzi, E.; Meinhardt, M.; Skondra, C.; Mannelli, M.; Robledo, M.; et al. Epigenetic Mutation of the Succinate Dehydrogenase C Promoter in a Patient With Two Paragangliomas. J. Clin. Endocrinol. Metab. 2016, 101, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbini, M.; Astolfi, A.; Indio, V.; Heinrich, M.C.; Corless, C.L.; Nannini, M.; Ravegnini, G.; Biasco, G.; Pantaleo, M.A. SDHC methylation in gastrointestinal stromal tumors (GIST): A case report. BMC Med. Genet. 2015, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, F.; Moskalev, E.A.; Faucz, F.R.; Barthelmeß, S.; Wiemann, S.; Bieg, M.; Assié, G.; Bertherat, J.; Schaefer, I.-M.; Otto, C.; et al. Aberrant DNA hypermethylation of SDHC: A novel mechanism of tumor development in Carney triad. Endocr.-Relat. Cancer 2014, 21, 567–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemoeller, O.M.; Niyazi, M.; Corradini, S.; Zehentmayr, F.; Li, M.; Lauber, K.; Belka, C. MicroRNA expression profiles in human cancer cells after ionizing radiation. Radiat. Oncol. 2011, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puissegur, M.-P.; Mazure, N.M.; Bertero, T.; Pradelli, L.; Grosso, S.; Robbe-Sermesant, K.; Maurin, T.; Lebrigand, K.; Cardinaud, B.; Hofman, V.; et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2010, 18, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Merlo, A.; De Quiros, S.B.; Secades, P.; Zambrano, I.; Balbín, M.; Astudillo, A.; Scola, B.; Arístegui, M.; Suarez, C.; Chiara, M.-D. Identification of a Signaling Axis HIF-1α/MicroRNA-210/ISCU Independent of SDH Mutation That Defines a Subgroup of Head and Neck Paragangliomas. J. Clin. Endocrinol. Metab. 2012, 97, E2194–E2200. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Mantel, C.; Lee, S.A.; Moon, S.-H.; Broxmeyer, H.E. MiR-31/SDHA Axis Regulates Reprogramming Efficiency through Mitochondrial Metabolism. Stem Cell Rep. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eichner, L.J.; Perry, M.-C.; Dufour, C.R.; Bertos, N.; Park, M.; St-Pierre, J.; Giguère, V. miR-378∗ Mediates Metabolic Shift in Breast Cancer Cells via the PGC-1β/ERRγ Transcriptional Pathway. Cell Metab. 2010, 12, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Baysal, B.E.; De Jong, K.; Liu, B.; Wang, J.; Patnaik, S.K.; Wallace, P.K.; Taggart, R.T. Hypoxia-inducible C-to-U coding RNA editing downregulatesSDHBin monocytes. PeerJ 2013, 1, e152. [Google Scholar] [CrossRef] [Green Version]
- Garaude, J.; Acín-Pérez, R.; Martínez-Cano, S.; Enamorado, M.; Ugolini, M.; Nistal-Villán, E.; Hervás-Stubbs, S.; Pelegrín, P.; Sander, L.E.; Enríquez, J.A.; et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016, 17, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.K.; Ryu, J.H.; Jin, Y.N.; Roberts, L.D.; Dejam, A.; Gerszten, R.E.; Peterson, R.T. PTPMT1 Inhibition Lowers Glucose through Succinate Dehydrogenase Phosphorylation. Cell Rep. 2015, 10, 694–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, L.W.S.; Haas, W.; Desquiret-Dumas, V.; Wallace, D.C.; Procaccio, V.; Gygi, S.P.; Haigis, M.C. Succinate Dehydrogenase Is a Direct Target of Sirtuin 3 Deacetylase Activity. PLoS ONE 2011, 6, e23295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-T.; Huang, D.; Shen, S.; Cai, Y.; Xing, S.; Wu, G.; Jiang, Z.; Hao, Y.; Yuan, M.; Wang, N.; et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat. Metab. 2020, 2, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Su, X.; He, B. Protein Lysine Acylation and Cysteine Succination by Intermediates of Energy Metabolism. ACS Chem. Biol. 2012, 7, 947–960. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.; Dai, L.; Xie, Z.; Zhang, Y.; Zwaans, B.M.; Skinner, M.E.; et al. SIRT5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.E.; Xu, H.; Chen, H.-L.; Chen, W.; Denton, T.T.; Zhang, S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J. Neurochem. 2015, 134, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef] [Green Version]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pell, V.R.; Spiroski, A.-M.; Mulvey, J.; Burger, N.; Costa, A.; Logan, A.; Gruszczyk, A.V.; Rosa, T.; James, A.M.; Frezza, C.; et al. Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J. Mol. Cell. Cardiol. 2018, 123, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.T.; Miller, J.H.; Day, M.M.; Munger, J.C.; Brookes, P.S. Accumulation of Succinate in Cardiac Ischemia Primarily Occurs via Canonical Krebs Cycle Activity. Cell Rep. 2018, 23, 2617–2628. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, Y.-L.; Li, L.-Z.; Zhang, L.; Liu, Q.; Liu, K.; Li, P.; Liu, B.; Qi, L.-W. Succinate accumulation impairs cardiac pyruvate dehydrogenase activity through GRP91-dependent and independent signaling pathways: Therapeutic effects of ginsenoside Rb1. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Increased Succinate Accumulation Induces ROS Generation in In Vivo Ischemia/Reperfusion-Affected Rat Kidney Mitochondria. BioMed. Res. Int. 2020, 2020, 8855585. [Google Scholar] [CrossRef]
- Gu, C.; Yang, H.; Chang, K.; Zhang, B.; Xie, F.; Ye, J.; Chang, R.; Qiu, X.; Wang, Y.; Qu, Y.; et al. Melatonin alleviates progression of uterine endometrial cancer by suppressing estrogen/ubiquitin C/SDHB-mediated succinate accumulation. Cancer Lett. 2020, 476, 34–47. [Google Scholar] [CrossRef]
- Wijermars, L.G.; Schaapherder, A.F.; Kostidis, S.; Wust, R.C.; Lindeman, J.H. Succinate Accumulation and Ischemia–Reperfusion Injury: Of Mice but Not Men, a Study in Renal Ischemia–Reperfusion. Am. J. Transplant. 2016, 16, 2741–2746. [Google Scholar] [CrossRef] [Green Version]
- Kluckova, K.; Tennant, D.A. Metabolic implications of hypoxia and pseudohypoxia in pheochromocytoma and paraganglioma. Cell Tissue Res. 2018, 372, 367–378. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, E.D.; Selak, M.A.; Tennant, D.A.; Payne, L.J.; Crosby, S.; Frederiksen, C.M.; Watson, D.G.; Gottlieb, E. Cell-Permeating α-Ketoglutarate Derivatives Alleviate Pseudohypoxia in Succinate Dehydrogenase-Deficient Cells. Mol. Cell. Biol. 2007, 27, 3282–3289. [Google Scholar] [CrossRef] [Green Version]
- Guzy, R.D.; Sharma, B.; Bell, E.; Chandel, N.S.; Schumacker, P.T. Loss of the SdhB, but Not the SdhA, Subunit of Complex II Triggers Reactive Oxygen Species-Dependent Hypoxia-Inducible Factor Activation and Tumorigenesis. Mol. Cell. Biol. 2008, 28, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ishii, K.-A.; Aita, Y.; Ikeda, T.; Kawakami, Y.; Shimano, H.; Hara, H.; Takekoshi, K. Loss of SDHB Elevates Catecholamine Synthesis and Secretion Depending on ROS Production and HIF Stabilization. Neurochem. Res. 2015, 41, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T.; Görlach, A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin. Cell Dev. Biol. 2005, 16, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Durán, R.V.; Gottlieb, E. Redox stress is not essential for the pseudo-hypoxic phenotype of succinate dehydrogenase deficient cells. Biochim. Biophys. Acta 2006, 1757, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, P.-L.; Wu, W.-H.; Hu, T.-H.; Chen, C.-W.; Cheng, H.-C.; Li, C.-F.; Tsai, W.-H.; Tsai, H.-J.; Hsieh, M.-C.; Chuang, J.-H.; et al. Decreased succinate dehydrogenase B in human hepatocellular carcinoma accelerates tumor malignancy by inducing the Warburg effect. Sci. Rep. 2018, 8, 3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.N.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef]
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.-P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 2004, 6, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Karshovska, E.; Wei, Y.; Subramanian, P.; Mohibullah, R.; Geißler, C.; Baatsch, I.; Popal, A.; Campos, J.C.; Exner, N.; Schober, A. HIF-1α (Hypoxia-Inducible Factor-1α) Promotes Macrophage Necroptosis by Regulating miR-210 and miR-383. Arter. Thromb. Vasc. Biol. 2020, 40, 583–596. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Lian, G.; Zhang, S.-Y.; Wang, X.; Jiang, C. HIF1α-Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Mediat. Inflamm. 2017, 2017, 9029327. [Google Scholar] [CrossRef] [Green Version]
- Hollander, A.P.; Corke, K.P.; Freemont, A.J.; Lewis, C.E. Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: Implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 2001, 44, 1540–1544. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.-Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.-R.; et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Wobben, R.; Hüsecken, Y.; Lodewick, C.; Gibbert, K.; Fandrey, J.; Winning, S. Role of hypoxia inducible factor-1α for interferon synthesis in mouse dendritic cells. Biol. Chem. 2013, 394, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Alexander, S.P.H.; Sharman, J.L.; Pawson, A.J.; Benson, H.E.; Monaghan, A.E.; Liew, W.C.; Mpamhanga, C.P.; Bonner, T.I.; Neubig, R.R.; et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G Protein-Coupled Receptor List: Recommendations for New Pairings with Cognate Ligands. Pharmacol. Rev. 2013, 65, 967–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wettschureck, N.; Offermanns, S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberger, T.; Schaller, H.; Hellebrand, S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J. Mol. Biol. 2001, 307, 799–813. [Google Scholar] [CrossRef]
- He, W.; Miao, F.J.-P.; Lin, D.C.-H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.-L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Geubelle, P.; Gilissen, J.; Dilly, S.; Poma, L.; Dupuis, N.; Laschet, C.; Abboud, D.; Inoue, A.; Jouret, F.; Pirotte, B.; et al. Identification and pharmacological characterization of succinate receptor agonists. J. Cereb. Blood Flow Metab. 2017, 174, 796–808. [Google Scholar] [CrossRef] [Green Version]
- Bhuniya, D.; Umrani, D.; Dave, B.; Salunke, D.; Kukreja, G.; Gundu, J.; Naykodi, M.; Shaikh, N.S.; Shitole, P.; Kurhade, S.; et al. Discovery of a potent and selective small molecule hGPR91 antagonist. Bioorg. Med. Chem. Lett. 2011, 21, 3596–3602. [Google Scholar] [CrossRef]
- Haffke, M.; Fehlmann, D.; Rummel, G.; Boivineau, J.; Duckely, M.; Gommermann, N.; Cotesta, S.; Sirockin, F.; Freuler, F.; Littlewood-Evans, A.; et al. Structural basis of species-selective antagonist binding to the succinate receptor. Nature 2019, 574, 581–585. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Zhao, Y.; Zhang, Y.; Li, H.; Zhang, A.; Wang, X.; Wang, W.; Hou, Y.; Wang, J. Succinate/IL-1β Signaling Axis Promotes the Inflammatory Progression of Endothelial and Exacerbates Atherosclerosis. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Trauelsen, M.; Hiron, T.K.; Lin, D.; Petersen, J.E.; Breton, B.; Husted, A.S.; Hjorth, S.A.; Inoue, A.; Frimurer, T.M.; Bouvier, M.; et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 2021, 35, 109246. [Google Scholar] [CrossRef] [PubMed]
- Robben, J.H.; Fenton, R.A.; Vargas, S.L.; Schweer, H.; Peti-Peterdi, J.; Deen, P.M.; Milligan, G. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009, 76, 1258–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakak, Y.; Lehmann-Bruinsma, K.; Phillips, S.; Le, T.; Liaw, C.; Connolly, D.T.; Behan, D.P. The role of the GPR91 ligand succinate in hematopoiesis. J. Leukoc. Biol. 2009, 85, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Högberg, C.; Gidlöf, O.; Tan, C.; Svensson, S.; Nilsson-Öhman, J.; Erlinge, D.; Olde, B. Succinate independently stimulates full platelet activation via cAMP and phosphoinositide 3-kinase-β signaling. J. Thromb. Haemost. 2011, 9, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Gnana-Prakasam, J.P.; Ananth, S.; Prasad, P.D.; Zhang, M.; Atherton, S.S.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Expression and Iron-Dependent Regulation of Succinate Receptor GPR91 in Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2011, 52, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Sundström, L.; Greasley, P.J.; Engberg, S.; Wallander, M.; Ryberg, E. Succinate receptor GPR91, a Gαicoupled receptor that increases intracellular calcium concentrations through PLCβ. FEBS Lett. 2013, 587, 2399–2404. [Google Scholar] [CrossRef] [Green Version]
- Gilissen, J.; Geubelle, P.; Dupuis, N.; Laschet, C.; Pirotte, B.; Hanson, J. Forskolin-free cAMP assay for Gi-coupled receptors. Biochem. Pharmacol. 2015, 98, 381–391. [Google Scholar] [CrossRef]
- Keiran, N.; Ceperuelo-Mallafré, V.; Calvo, E.; Hernández-Alvarez, M.I.; Ejarque, M.; Núñez-Roa, C.; Horrillo, D.; Maymó-Masip, E.; Rodríguez, M.M.; Fradera, R.; et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 2019, 20, 581–592. [Google Scholar] [CrossRef]
- Rubic, T.; Lametschwandtner, G.; Jost, S.; Hinteregger, S.; Kund, J.; Carballido-Perrig, N.; Schwärzler, C.; Junt, T.; Voshol, H.; Meingassner, J.G.; et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 2008, 9, 1261–1269. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Q.; Li, T.; Chen, Y.; Wang, S. Inhibition of high glucose-induced VEGF release in retinal ganglion cells by RNA interference targeting G protein-coupled receptor 91. Exp. Eye Res. 2013, 109, 31–39. [Google Scholar] [CrossRef]
- Aguiar, C.J.; Andrade, V.L.; Gomes, E.R.M.; Alves, M.N.M.; Ladeira, M.S.; Pinheiro, A.C.N.; Gomes, D.A.; Almeida, A.P.; Goes, A.M.; Resende, R.R.; et al. Succinate modulates Ca2+ transient and cardiomyocyte viability through PKA-dependent pathway. Cell Calcium 2010, 47, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzak, G.; Willis, C.M.; Smith, J.A.; Pluchino, S.; Peruzzotti-Jametti, L. Succinate Receptor 1: An Emerging Regulator of Myeloid Cell Function in Inflammation. Trends Immunol. 2020, 42, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Southern, C.; Cook, J.M.; Neetoo-Isseljee, Z.; Taylor, D.L.; Kettleborough, C.A.; Merritt, A.; Bassoni, D.L.; Raab, W.J.; Quinn, E.; Wehrman, T.S.; et al. Screening β-Arrestin Recruitment for the Identification of Natural Ligands for Orphan G-Protein–Coupled Receptors. J. Biomol. Screen. 2013, 18, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilissen, J.; Jouret, F.; Pirotte, B.; Hanson, J. Insight into SUCNR1 (GPR91) structure and function. Pharmacol. Ther. 2016, 159, 56–65. [Google Scholar] [CrossRef]
- Gimenez-Roqueplo, A.-P.; Favier, J.; Rustin, P.; Mourad, J.-J.; Plouin, P.-F.; Corvol, P.; Rötig, A.; Jeunemaitre, X. The R22X Mutation of the SDHD Gene in Hereditary Paraganglioma Abolishes the Enzymatic Activity of Complex II in the Mitochondrial Respiratory Chain and Activates the Hypoxia Pathway. Am. J. Hum. Genet. 2001, 69, 1186–1197. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Roqueplo, A.-P.; Favier, J.; Rustin, P.; Rieubland, C.; Kerlan, V.; Plouin, P.-F.; Rötig, A.; Jeunemaitre, X. Functional Consequences of a SDHB Gene Mutation in an Apparently Sporadic Pheochromocytoma. J. Clin. Endocrinol. Metab. 2002, 87, 4771–4774. [Google Scholar] [CrossRef] [Green Version]
- Pollard, P.J.; Briere, J.J.; Alam, N.A.; Barwell, J.; Barclay, E.; Wortham, N.C.; Hunt, T.; Mitchell, M.; Olpin, S.; Moat, S.J.; et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 2005, 14, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.-T.; Lin, Y.-T.; Tang, S.-P.; Luo, C.-K.; Tsai, C.-T.; Shun, C.-T.; Chen, C.-C. Metabolic targeting of HIF-1α potentiates the therapeutic efficacy of oxaliplatin in colorectal cancer. Oncogene 2019, 39, 414–427. [Google Scholar] [CrossRef]
- Louphrasitthiphol, P.; Ledaki, I.; Chauhan, J.; Falletta, P.; Siddaway, R.; Buffa, F.M.; Mole, D.R.; Soga, T.; Goding, C.R. MITF controls the TCA cycle to modulate the melanoma hypoxia response. Pigment. Cell Melanoma Res. 2019, 32, 792–808. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Kouskoukis, C.; Gatter, K.C.; Harris, A.; Koukourakis, M.I. Hypoxia-inducible factors 1alpha and 2alpha are related to vascular endothelial growth factor expression and a poorer prognosis in nodular malignant melanomas of the skin. Melanoma Res. 2003, 13, 493–501. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, G.; Stewart, R.L.; Chen, J.; Gao, T.; Scott, T.L.; Samayoa, L.M.; O’Connor, K.; Lane, A.N.; Xu, R. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat. Commun. 2018, 9, 4456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.-S.; Cho, J.-H.; Honoré, J.-C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Hamel, D.; Bajon, E.; Duhamel, F.; Bhosle, V.K.; Zhu, T.; Rivera, J.C.; Dabouz, R.; Nadeau-Vallée, M.; Sitaras, N.; et al. The Succinate Receptor SUCNR1 Resides at the Endoplasmic Reticulum and Relocates to the Plasma Membrane in Hypoxic Conditions. Cells 2022, 11, 2185. [Google Scholar] [CrossRef] [PubMed]
- Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Bouhlel, A.; Hache, G.; Dignat-George, F.; Taïeb, D.; Guillet, B. Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study. Cells 2021, 10, 795. [Google Scholar] [CrossRef]
- Hamel, D.; Sanchez, M.; Duhamel, F.; Roy, O.; Honoré, J.-C.; Noueihed, B.; Zhou, T.; Nadeau-Vallée, M.; Hou, X.; Lavoie, J.-C.; et al. G-Protein–Coupled Receptor 91 and Succinate Are Key Contributors in Neonatal Postcerebral Hypoxia-Ischemia Recovery. Arter. Thromb. Vasc. Biol. 2014, 34, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Kes, M.M.; Bossche, J.V.D.; Griffioen, A.W.; Huijbers, E.J. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1874, 188427. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [Green Version]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Menga, A.; Martín-Pérez, R.; Quinto, A.; Riera-Domingo, C.; De Tullio, G.; Hooper, D.C.; Lamers, W.H.; Ghesquière, B.; McVicar, D.W.; et al. Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 2017, 20, 1654–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, V.; Eykyn, T.R.; Mustapha, R.; Flores-Borja, F.; Male, V.; Barber, P.R.; Patsialou, A.; Green, R.; Panagaki, F.; Li, C.W.; et al. Breast cancer–associated macrophages promote tumorigenesis by suppressing succinate dehydrogenase in tumor cells. Sci. Signal. 2020, 13, eaax4585. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atallah, R.; Olschewski, A.; Heinemann, A. Succinate at the Crossroad of Metabolism and Angiogenesis: Roles of SDH, HIF1α and SUCNR1. Biomedicines 2022, 10, 3089. https://doi.org/10.3390/biomedicines10123089
Atallah R, Olschewski A, Heinemann A. Succinate at the Crossroad of Metabolism and Angiogenesis: Roles of SDH, HIF1α and SUCNR1. Biomedicines. 2022; 10(12):3089. https://doi.org/10.3390/biomedicines10123089
Chicago/Turabian StyleAtallah, Reham, Andrea Olschewski, and Akos Heinemann. 2022. "Succinate at the Crossroad of Metabolism and Angiogenesis: Roles of SDH, HIF1α and SUCNR1" Biomedicines 10, no. 12: 3089. https://doi.org/10.3390/biomedicines10123089